A Multi-Spectroscopic Study for the Characterization and Definition of Production Techniques of German Ceramic Sherds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

If Sherds Could Tell Imported C
Everyday Products in the Middle Ages Ages Middle the in Products Everyday The medieval marketplace is a familiar setting in popular and academic Everyday Products accounts of the Middle Ages, but we actually know very little about the people involved in the transactions that took place there, and how their lives were influenced by those transactions. We know still less about the in the Middle Ages complex networks of individuals whose actions allowed raw materials to be extracted, hewn into objects, stored and ultimately shipped for market. Crafts, Consumption and the Individual With these elusive individuals in mind, this volume will explore the worlds of actors involved in the lives of objects. We are particularly concerned in Northern Europe c. AD 800–1600 with everyday products - objects of bone, leather, stone, ceramics, and base metal - their production and use in medieval northern Europe. The volume brings together 20 papers, first presented at the event ‘Actors and Affordable Crafts: Social and Economic Networks in Medieval northern Europe’, organised by the Universities of Bergen and York in February 2011. Through diverse case studies undertaken by specialists, and a combination of leading edge techniques and novel theoretical approaches, we aim to illuminate the identities and lives of the medieval period’s oft-overlooked actors. This collection then, does not engage directly with the traditional foci of research into medieval crafts - questions of economics, politics, or technological development - but rather takes a social approach. Neither are we concerned with the writing of a grand historical narrative, but rather with the painting of a number of detailed portraits, which together may prove far more illuminating than any generalising broadbrush approach and Irene Baug Ashby P. -

Informationsveranstaltung Schuljahr 2020-21
Informationsveranstaltungen der weiterführenden Schulen in Trägerschaft des Landkreises Hildesheim für die künftigen 5. Klassen des Schuljahres 2020/21 Schulform Schule Ort Datum Uhrzeit Art "Markt der Bildungsmöglichkeiten" gemeinsam mit der Realschule 29.11.2019 16.00 bis 18.00 Uhr Hauptschule Schulrat‐Habermalz‐Schule Alfeld und Gymnasium Alfeld (im Forum Schulrat‐Habermalz‐Schule) 11.02. und 12.02.2020 Vormittags Schnuppertag "Markt der Bildungsmöglichkeiten"gemeinsam mit der Hauptschule und 29.11.2019 16.00 bis 18.00 Uhr Gymnasium Alfeld (im Forum Schulrat‐Habermalz‐Schule) RealschuleCarl‐Benscheidt‐Realschule Alfeld 09.12.2019 Vormittags "Schnupperunterricht" für Schüler der Alfelder Grundschulen 10.12.2019 Vormittags "Schnupperunterricht" für Grundschüler aus umliegenden Ortschaften 06.03.2020 16.00 bis 18.00 Uhr Tag der offenen Tür 04.12.2019 18.00 bis 19.00 Uhr Informationsveranstaltung Oberschule Lamspringe Lamspringe 17.01.2020 8.00 bis 12.00 Uhr Schnuppertag 06.02 und 07.02.2020 Vormittags Schnupperunterricht Marienbergschule Nordstemmen 06.02.2020 19.00 Uhr Info‐Veranstaltung für die Eltern Molitoris‐Schule Harsum 04.12.2019 17.00 Uhr Informationsabend 15.11.2019 auf Einladung Vorlesetag für Grundschüer Oberschule Bockenem Bockenem 29.11.2019 16.00 bis 19.00 Uhr Adventsbasar 20.02.2020 15.00 bis 17.00 Uhr Kennenlerntag für Grundschüler 05.11.2019 19.30 Uhr Infoveranstaltung Oberschule Schiller‐Oberschule Sarstedt Termin in Schule erfragen Vormittags "Schnuppertag" für die Grundschule Regenbogenschule Termin in Schule erfragen -

Standor Tinforma Tion
Die Stadt Bad Salzdetfurth mit ca. 14.500 Einwohnern, als Bestandteil der Region Hildesheim, liegt zwischen den international bedeutsamen Wirtschaftszentren Hannover und Braunschweig-Wolfsburg sowie im Zentrum der renommierten Hochschul- und Forschungs- regionen Hannover, Braunschweig-Wolfenbüttel, Göttingen und Clausthal-Zellerfeld. Damit befindet sie sich im Mittelpunkt der größten Wirtschafts- und Bevöl- kerungsagglomeration in Niedersachsen. In der Wirtschaftsregion Hildesheim haben sich aufgrund der sehr günstigen Rahmenbedingungen in den letzten Jahren mittelständische Firmen unterschiedlichster STANDORTINFORMATION Branchen angesiedelt. International angesehene Unter- nehmen der Elektrotechnik, der Nachrichten- und Über- tragungstechnik, des Maschinen- und Apparatebaus, der Papierherstellung und -verarbeitung, des Modell- und Formenbaus und der Gummiindustrie. Eine Vielfalt von Handwerks- und Handelsunternehmen und insbesondere die Dienstleistungsbetriebe – sprich Großhandel, Verkehrs- und Nachrichtenwesen, Kreditinstitute, Versicherungen, Krankenhäuser und Heime – runden dieses starke Erscheinungsbild ab. Die Wirtschaftsregion Hildesheim ist als Fördergebiet nach der Gemeinschaftsaufgabe „Verbesserung der regionalen Wirtschaftsstruktur“ ausgewiesen. Im Dezember 2000 wurde das TecCenter Bad Salzdet- Hannover Messe furth von der TecCenter Grundstücksentwicklungs- Mittellandkanal Hannover gesellschaft mbH & Co. KG gegründet. Gesellschafter DB-Neubaustrecke sind die Kreissparkasse Hildesheim und die Stadt Bad Hannover-Würzburg mit -

Organigramm Des Kirchenamtes Hildesheim Telefon: 05121 100-0 Telefax: 05121 100-999 (Sekretariat OG)
Verwaltungsleitung Jens Stöber, Tel. -600 Querschnittsaufgaben Organigramm des Kirchenamtes Hildesheim Monika Reicke*) / Andrea Hildebrandt / Sabine Reich-Meyer*) Stand: 01.01.2018 · Leitung Kirchenamt Tel. -601 / -603 / -601 Telefon: 05121 100-0 · Betreuung Verbandsvorstand · Postausgang / · Posteingang Telefax: 05121 100-999 (Sekretariat OG) · Erteilung von Genehmigungen nach KGO und KKO sowie · Registratur/Archiv / · Sitzungsdienst Beanstandung von Beschlüssen · Telefonzentrale · Budgetplanung Datenschutzbeauftragte: Elke Schünemann * -106 · Betreuung Kirchenkreisverband Hildesheim Fachbereich I Fachbereich II Fachbereich III Fachbereich IV Fachbereich V - Finanz- und Bauwesen - Rechnungswesen – - Liegenschaften / Friedhöfe / EDV / - Personalwesen – - Diakonie - Fachbereichsleiter: Hartmut Haase Meldewesen Fachbereichsleiter: Helmut Jost Tel. -200 Fachbereichsleiter: Matthias Wehling Fachbereichsleiter: Sven Böning Fachbereichsleiterin: Cordula Stepper Tel. -100 · Leitung des Fachbereiches Tel. -300 Tel. -400 Tel. -500 · stellvertretende Verwaltungsleitung · Verwaltung Rücklagen- u. Darlehensfonds (RDF) · Leitung des Fachbereiches · Leitung des Fachbereiches · stellvertretende Verwaltungsleitung · Leitung des Fachbereiches · Disposition der Geldanlagen · Generalia Liegenschaften und · grundsätzliche Fragen des · Leitung des Fachbereiches · Finanzplanung/Gesamtzuweisung Kirchenkreisverband · Aufsicht über Zahlstellen Ersatzlanderwerbe, EDV, Meldewesen Arbeits- und Dienstrechtes · Betreuung · Betreuung Kirchenkreisvorstand und FiPla-Ausschuss -

Haushaltsverteilung
Haushaltsverteilung Verteilgebiet: Hildesheim Landkreis/kreisfreie Stadt Postleitzahl Stadtteil/Ort Aufl age Elze 31008 Elze 2.320 Elze 31008 Esbeck 70 Elze 31008 Mehle 555 Elze 31008 Sehlde 200 Elze 31008 Sorsum 120 Elze 31008 Wittenburg 40 Elze 31008 Wülfi ngen 270 Gronau 31028 Dötzum 45 Gronau 31029 Gronau 2.245 Banteln 31029 Banteln 660 Betheln 31032 Betheln 420 Betheln 31032 Eddinghausen 50 Betheln 31032 Haus Escherde 20 Brüggen 31033 Brüggen 460 Despetal 31035 Barfelde 300 Despetal 31035 Eitzum 250 Despetal 31035 Nienstedt 66 Eime 31036 Deilmissen 120 Eime 31036 Deinsen 115 Eime 31036 Dunsen 23 Eime 31036 Eime 935 Eime 31036 Heinsen 17 Rheden 31039 Heinum 60 Rheden 31039 Rheden 225 Rheden 31039 Wallenstedt 140 Alfeld 31061 Alfeld 6.204 Alfeld 31061 Brunkensen 400 Alfeld 31061 Dehnsen 300 Alfeld 31061 Eimsen 310 Alfeld 31061 Föhrste 465 Alfeld 31061 Godenau 122 Alfeld 31061 Gerzen 489 Alfeld 31061 Hörsum 290 Alfeld 31061 Imsen 140 Alfeld 31061 Langenholzen 566 Alfeld 31061 Limmer 340 Alfeld 31061 Lütgenholzen 20 Alfeld 31061 Röllinghausen 116 Alfeld 31061 Sack 250 Alfeld 31061 Warzen 200 Alfeld 31061 Wettensen 42 Alfeld 31061 Wispenstein 140 Adenstedt 94065 Adenstedt 290 Adenstedt 94065 Grafelde 110 Adenstedt 94065 Sellenstedt 100 Haushaltsverteilung Landkreis/kreisfreie Stadt Postleitzahl Stadtteil/Ort Aufl age Almstedt 31079 Almstedt 350 Almstedt 31079 Segeste 100 Eberholzen 31079 Eberholzen 265 Sibbesse 31079 Hönze 200 Sibbesse 31079 Möllensen 60 Sibbesse 31079 Petze 250 Sibbesse 31079 Sibbesse 925 Westfeld 31079 Westfeld 235 Westfeld -
Samtgemeinde Duingen Landkreis Hildesheim Gemarkung Weenzen
6 5/ ,2 0 Ah 3 5 5 3 5 5 5 5 5 5 5 5 4 4 6 5 6 4 5 0 7 7 7 7 7 7 0 7 7 0 0 AcGhsraed.== 6 0G1_ W W 7 n 6 6 6 6 6 6 6 6 Q e 6 4 4 4 4 4 4 5 z 5 5 6 .035 n 4 5 6 7 Km= 60+325 8 9 1 2 0 B e 0 E 135/2 72 N 0 0 0 = 60_ WW 0 0 0 ( 0 0 Achse = G1 G rad. e + 00 78 k 5740 H = 35 0 A m 0 0 0 2 0 0 0 0 0 V W C022 _ a Achse = 01 n 4 G rad. = G6 0 o 1 67 t = 12.438 1 48/20 2 g i 2. h 2 24 n 1 e 0+ 6 u c m= Acker 43 d r , , K e 73 9 5 = 0.022 a hw 0 f d 0 s n 6 20 - 35 H = 26 w e 044.0 Q 2 = 2+ S 190.556 m T k 1 K s 56 c . 9 f Q ei = 2 = t 2 Acker / Q m o d 5780 Q + Q 0 8 00 h 1 10 de Q = 0.2 H = 2 f h 1 M + 2 e 6 4 2 76 6 . l 1 9 d 8 + 1 2 W 0_W + 0 se= 6 1 S 8 Ach d. = G 8 B G ra T B 4.5 0 201/71 2 u + = 1 t 3 0 r + e 2 667% 3 s =1. -
Um-Maps---G.Pdf
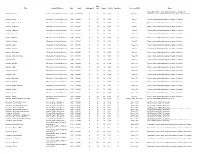
Map Title Author/Publisher Date Scale Catalogued Case Drawer Folder Condition Series or I.D.# Notes Topography, towns, roads, political boundaries for parts of Gabon - Libreville Service Géographique de L'Armée 1935 1:1,000,000 N 35 10 G1-A F One sheet Cameroon, Gabon, all of Equatorial Guinea, Sao Tomé & Principe Gambia - Jinnak Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 1 Towns, roads, political boundaries for parts of Gambia Gambia - N'Dungu Kebbe Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 2 Towns, roads, political boundaries for parts of Gambia Gambia - No Kunda Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 4 Towns, roads, political boundaries for parts of Gambia Gambia - Farafenni Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 5 Towns, roads, political boundaries for parts of Gambia Gambia - Kau-Ur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 Towns, roads, political boundaries for parts of Gambia Gambia - Bulgurk Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 A Towns, roads, political boundaries for parts of Gambia Gambia - Kudang Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 Towns, roads, political boundaries for parts of Gambia Gambia - Fass Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 A Towns, roads, political boundaries for parts of Gambia Gambia - Kuntaur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 8 Towns, roads, political -

Niedersächsisches Justizministerium
Ahlden (Aller) Haßbergen Grethem Eickeloh Hankensbüttel Rohrsen Steinhorst Hademstorf Winsen Heemsen Heemsen Essel (Aller) Dedelstorf Gilten WITTINGEN Schwarmstedt CELLE Rodewald Eldingen Steimbke Schwarm- stedt Buchholz (Aller) Beeden- Steimbke bostel NIENBURG Wietze Lachendorf Groß Oesingen Schöne- Lachendorf wörde Stöckse Hambühren Lindwedel Wesendorf Flecken Ahnsbeck Wahrenholz Brome Bezirk des Oberlandesgerichts Hohne Wesendorf Ehra-Lessien Tülau Linsburg Ummern Wienhausen Brome und der Generalstaatsanwaltschaft Husum Niedersachsen Adelheidsdorf Gifhorn Nienburg/Weser Wagen- Braunschweig Gemeindegrenzenkarte Wathlingen Flotwedel Langlingen hoff Wedemark Bergfeld NEUSTADT Parsau Nien- Eicklingen am Rübenberge Barwedel - Organisation der ordentlichen Gerichte hagen Sassenburg BURGWEDEL Wath- lingen Müden und Staatsanwaltschaften - Region Bröckel (Aller) Boldecker Land Grenze eines Bundeslandes GIFHORN Tiddische Bokens- Jembke dorf Rühen Grenze eines Nds. Landkreises Tappen- Meinersen Osloß beck Stand: 1. September 2015 Grenze einer Nds. Einheitsgemeinde / Samtgemeinde Meinersen Wey- LANGENHAGEN BURGDORF hausen GARBSEN Isernhagen Uetze Isenbüttel Graf- Grenze einer Nds. Mitgliedsgemeinde horst Leiferde Isenbüttel Dann- Ribbesbüttel dorf Calberlah Flecken Gemeindefreies Gebiet in Niedersachsen Hagenburg WUNSTORF Velpke Wölping- Hillerse Wasbüttel WOLFSBURG hausen Peine Name eines Nds. Landkreises Rötgesbüttel Sachsenhagen Edemissen Velpke SACHSEN- (Peine) Au- WOLFSBURG Name einerLandeshauptstadt Kreisfreien Stadt Adenbüttel HAGEN hagen -

17. Wahlperiode Drucksache 17/4742 1
Niedersächsischer Landtag 17. Wahlperiode Drucksache 17/4742 Beschlussempfehlung Ausschuss Hannover, den 03.12.2015 für Inneres und Sport Entwurf eines Gesetzes über die Vereinigung der Gemeinden Banteln, Betheln, Brüggen, Despetal, Rheden und der Stadt Gronau (Leine) sowie über die Neubildung des Fleckens Duingen und der Samtgemeinde Leinebergland, Landkreis Hildesheim Gesetzentwurf der Landesregierung - Drs. 17/4488 Berichterstatter: Abg. Bernd Lynack (SPD) (Es ist ein schriftlicher Bericht vorgesehen.) Der Ausschuss für Inneres und Sport empfiehlt dem Landtag, den Gesetzentwurf mit den aus der Anlage ersichtlichen Änderungen anzunehmen. Johann-Heinrich Ahlers Vorsitzender 1 Niedersächsischer Landtag – 17. Wahlperiode Drucksache 17/4742 Gesetzentwurf der Landesregierung - Drs. 17/4488 Empfehlungen des Ausschusses für Inneres und Sport Gesetz Gesetz über die Vereinigung der Gemeinden Banteln, über die Vereinigung der Gemeinden Banteln, Betheln, Brüggen, Despetal, Rheden und der Betheln, Brüggen, Despetal, Rheden und der Stadt Gronau (Leine) sowie über die Neubildung Stadt Gronau (Leine) sowie über die Neubildung des Fleckens Duingen und der Samtgemeinde des Fleckens Duingen und der Samtgemeinde Leinebergland, Landkreis Hildesheim Leinebergland, Landkreis Hildesheim § 1 § 1 (1) 1Die Gemeinden Banteln, Betheln, Brüggen, unverändert Despetal und Rheden sowie die Stadt Gronau (Leine) werden vereinigt, indem die Gemeinden Banteln, Betheln, Brüggen, Despetal und Rheden in die Stadt Gronau (Leine) eingegliedert werden. 2Zugleich werden die Gemeinden Banteln, Betheln, Brüggen, Despetal und Rheden aufgelöst. (2) 1Aus dem Flecken Duingen und den Gemein- den Coppengrave, Hoyershausen, Marienhagen und Weenzen wird der neue Flecken Duingen gebildet. 2Zugleich werden der bisherige Flecken Duingen und die Gemeinden Coppengrave, Hoyershausen, Marienhagen und Weenzen aufgelöst. (3) 1Aus der Stadt Gronau (Leine), dem neuen Fle- cken Duingen und dem Flecken Eime wird die Samtge- meinde Leinebergland gebildet. -

Moravian Ceramics on St Croix, the Virgin Islands
Post-Medieval Archaeology ISSN: 0079-4236 (Print) 1745-8137 (Online) Journal homepage: https://www.tandfonline.com/loi/ypma20 Moravian ceramics on St Croix, the Virgin Islands By Natascha Mehler, Torbjörn Brorsson, Jette Linaa & Richard Gartley To cite this article: By Natascha Mehler, Torbjörn Brorsson, Jette Linaa & Richard Gartley (2018): Moravian ceramics on St Croix, the Virgin Islands, Post-Medieval Archaeology, DOI: 10.1080/00794236.2018.1515413 To link to this article: https://doi.org/10.1080/00794236.2018.1515413 © Society for Post-Medieval Archaeology 2018 Published online: 24 Dec 2018. Submit your article to this journal Article views: 80 View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ypma20 Post-Medieval Archaeology (2018), 1–4 Notes and News Moravian ceramics on St Croix, the Virgin Islands By NATASCHA MEHLER, TORBJORN€ BRORSSON, JETTE LINAA and RICHARD GARTLEY The island of St Croix in the Caribbean Sea, today a The pottery was identified as originating from part of the United States Virgin Islands, is a cultural Bethlehem in Pennsylvania, where Moravian potters melting pot of descendants of European settlers, a produced slip-decorated redwares.3 The results are native population and African slaves. The English based on visual identification and a comparison of arrived in 1631, after which followed episodes of both the Pennsylvania and St Croix assemblages Spanish and French occupation until 1733, when the based upon morphology and decoration. Written island was sold to the Danish West India Company. sources from 1759 testify to the export of ceramics In 1734, Moravian missionaries arrived on the island from Bethlehem to St Croix.4 and they soon established three missionary stations Ten of the 50 sherds from St Croix were chosen in order to evangelize the African slaves who had for an ICP-MS analysis in an attempt to determine arrived on board the Danish ships.1 The missionaries, their origin (Fig. -

Infoflyer-Kdu-0816.Pdf
Umzug Wir sind für Sie da! Was ist zu tun bei einem Umzug? Bitte melden Sie sich mit dem konkreten Mietange- bot bei Ihrem Jobcenter Hildesheim. Damit Ihnen keine fi nanziellen Risiken entstehen, kann das Jobcenter Hildesheim vor Abschluss des neuen Mietvertrages prüfen, ob Ihnen eine Wer ist zuständig? Zusicherung zur Anmietung dieser Wohnung aus- gesprochen werden kann. Dies ist grundsätzlich der Sie möchten innerhalb des Landkreis Hildesheim Fall, wenn der Umzug erforderlich ist und die Auf- einschließlich Stadtgebiet Hildesheim umziehen wendungen für die neue Unterkunft angemessen oder möchten aus einem anderen Landkreis hierher sind. ziehen? Kosten der Unterkunft Dann ist das Jobcenter Hildesheim für Sie der richti- Darüber hinaus können Kosten für die Wohnungs- ge Ansprechpartner. Die konkreten Zuständigkeiten beschaffung (etwa eine Mietkaution oder Genos- fi nden Sie auf unserer Homepage. Umzugskosten senschaftsanteile) und Umzugskosten übernommen werden, wenn das Jobcenter Hildesheim dieses im Sofern Sie außerhalb des Landkreis Hildesheim eine Rahmen einer weiteren Zusicherung geprüft hat. Die- neue Unterkunft beziehen möchten, ist das Jobcen- se Kosten können wiederum übernommen werden, ter an diesem neuen Wohnort für Sie zuständig. wenn der Umzug durch das Jobcenter Hildesheim veranlasst oder aus anderen Gründen notwendig ist Wichtig ist in jedem Fall: und wenn ohne diese Zusicherung eine Unterkunft Setzen Sie sich bereits vor Abschluss eines neu- in einem angemessenem Zeitraum nicht gefunden en Mietvertrages unbedingt mit dem konkreten werden kann. Mietangebot mit Ihrem Jobcenter in Verbindung! Achtung! Wenn Sie unter 25 Jahre alt sind, kann eine Zusiche- rung der Kostenübernahme nur in besonderen Här- tefällen gewährt werden. Ohne diese vorherige Zusi- Jobcenter Hildesheim cherung des Jobcenters Hildesheim werden keinerlei Am Marienfriedhof 3 Kosten übernommen! Diese Regelung gilt sowohl bei 31134 Hildesheim einem erstmaligen Auszug aus der elterlichen Woh- nung, als auch bei jedem folgenden Umzug. -

Official Journal C 30 Volume 33 of the European Communities 8 Febraary 1990
yr-^^ /*/*• • "I "Y* 1 ISSN 0378-6986 Official Journal c 30 Volume 33 of the European Communities 8 Febraary 1990 English edition Information and Notices Notice No Contents Page I Information II Preparatory Acts Commission 90/C 30/01 Proposal for Council Directive amending Directive 86/465/EEC concerning the Community list of less-favoured farming areas within the meaning of Directive 75/ 268/EEC (Federal Republic of Germany) 1 90/C 30/02 Proposal for a Council Directive concerning the Community list of less-favoured farming areas within the meaning of Directive 75/268/EEC (France) 35 90/C 30/03 Proposal for Council Directive amending Directive 81/645/EEC concerning the Community list of less-favoured farming areas within the meaning of Directive 75/ 268/EEC (Greece) 50 90/C 30/04 Amended proposal for a Council Directive on the annual accounts and consolidated accounts of insurance undertakings 51 90/C 30/05 Re-examined proposal for a Council Directive amending Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations 89 90/C 30/06 Re-examined proposal for a Council Directive on company law concerning single- member private limited companies 91 90/C 30/07 Re-examined proposal for a Council Directive amending Directive 87/102/EEC of 22 December 1986 on the approximation of the laws, regulations and administrative provisions of the Member States concerning consumer credit 92 90/C 30/08 Re-examined proposal for a: — Council Directive amending Directive 86/298/EEC on rear-mounted roll-over protection structures narrow-track tractors — Council Directive amending Directive 87/402/EEC on roll-over protection structures mounted at the front of narrow-track tractors — Council Directive amending Directive 77/536/EEC on roll-over protection structures for tractors (standard) 95 Price: 12 ECU 8.