Archaeometric Studies of Historical Ceramic Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

If Sherds Could Tell Imported C
Everyday Products in the Middle Ages Ages Middle the in Products Everyday The medieval marketplace is a familiar setting in popular and academic Everyday Products accounts of the Middle Ages, but we actually know very little about the people involved in the transactions that took place there, and how their lives were influenced by those transactions. We know still less about the in the Middle Ages complex networks of individuals whose actions allowed raw materials to be extracted, hewn into objects, stored and ultimately shipped for market. Crafts, Consumption and the Individual With these elusive individuals in mind, this volume will explore the worlds of actors involved in the lives of objects. We are particularly concerned in Northern Europe c. AD 800–1600 with everyday products - objects of bone, leather, stone, ceramics, and base metal - their production and use in medieval northern Europe. The volume brings together 20 papers, first presented at the event ‘Actors and Affordable Crafts: Social and Economic Networks in Medieval northern Europe’, organised by the Universities of Bergen and York in February 2011. Through diverse case studies undertaken by specialists, and a combination of leading edge techniques and novel theoretical approaches, we aim to illuminate the identities and lives of the medieval period’s oft-overlooked actors. This collection then, does not engage directly with the traditional foci of research into medieval crafts - questions of economics, politics, or technological development - but rather takes a social approach. Neither are we concerned with the writing of a grand historical narrative, but rather with the painting of a number of detailed portraits, which together may prove far more illuminating than any generalising broadbrush approach and Irene Baug Ashby P. -

TRANSIENT LUNAR PHENOMENA: REGULARITY and REALITY Arlin P
The Astrophysical Journal, 697:1–15, 2009 May 20 doi:10.1088/0004-637X/697/1/1 C 2009. The American Astronomical Society. All rights reserved. Printed in the U.S.A. TRANSIENT LUNAR PHENOMENA: REGULARITY AND REALITY Arlin P. S. Crotts Department of Astronomy, Columbia University, Columbia Astrophysics Laboratory, 550 West 120th Street, New York, NY 10027, USA Received 2007 June 27; accepted 2009 February 20; published 2009 April 30 ABSTRACT Transient lunar phenomena (TLPs) have been reported for centuries, but their nature is largely unsettled, and even their existence as a coherent phenomenon is controversial. Nonetheless, TLP data show regularities in the observations; a key question is whether this structure is imposed by processes tied to the lunar surface, or by terrestrial atmospheric or human observer effects. I interrogate an extensive catalog of TLPs to gauge how human factors determine the distribution of TLP reports. The sample is grouped according to variables which should produce differing results if determining factors involve humans, and not reflecting phenomena tied to the lunar surface. Features dependent on human factors can then be excluded. Regardless of how the sample is split, the results are similar: ∼50% of reports originate from near Aristarchus, ∼16% from Plato, ∼6% from recent, major impacts (Copernicus, Kepler, Tycho, and Aristarchus), plus several at Grimaldi. Mare Crisium produces a robust signal in some cases (however, Crisium is too large for a “feature” as defined). TLP count consistency for these features indicates that ∼80% of these may be real. Some commonly reported sites disappear from the robust averages, including Alphonsus, Ross D, and Gassendi. -

Art: Authenticity, Restoration, Forgery
UCLA Cotsen Institute of Archaeology Press Title Art: Authenticity, Restoration, Forgery Permalink https://escholarship.org/uc/item/5xf6b5zd ISBN 978-1-938770-08-1 Author Scott, David A. Publication Date 2016-12-01 Data Availability The data associated with this publication are within the manuscript. Peer reviewed eScholarship.org Powered by the California Digital Library University of California READ ONLY/NO DOWNLOADS Art: Art: Authenticity, Restoration, ForgeryRestoration, Authenticity, Art: Forgery Authenticity, Restoration, Forgery David A. Scott his book presents a detailed account of authenticity in the visual arts from the Palaeolithic to the postmodern. The restoration of works Tof art can alter the perception of authenticity, and may result in the creation of fakes and forgeries. These interactions set the stage for the subject of this book, which initially examines the conservation perspective, then continues with a detailed discussion of what “authenticity” means, and the philosophical background. Included are several case studies that discuss conceptual, aesthetic, and material authenticity of ancient and modern art in the context of restoration and forgery. • Scott Above: An artwork created by the author as a conceptual appropriation of the original Egyptian faience objects. Do these copies possess the same intangible authenticity as the originals? Photograph by David A. Scott On front cover: Cast of author’s hand with Roman mask. Photograph by David A. Scott MLKRJBKQ> AO@E>BLILDF@> 35 MLKRJBKQ> AO@E>BLILDF@> 35 CLQPBK IKPQFQRQB LC AO@E>BLILDV POBPP CLQPBK IKPQFQRQB LC AO@E>BLILDV POBPP CIoA Press READ ONLY/NO DOWNLOADS Art: Authenticity, Restoration, Forgery READ ONLY/NO DOWNLOADS READ ONLY/NO DOWNLOADS Art: Authenticity, Restoration, Forgery David A. -

Water on the Moon, III. Volatiles & Activity
Water on The Moon, III. Volatiles & Activity Arlin Crotts (Columbia University) For centuries some scientists have argued that there is activity on the Moon (or water, as recounted in Parts I & II), while others have thought the Moon is simply a dead, inactive world. [1] The question comes in several forms: is there a detectable atmosphere? Does the surface of the Moon change? What causes interior seismic activity? From a more modern viewpoint, we now know that as much carbon monoxide as water was excavated during the LCROSS impact, as detailed in Part I, and a comparable amount of other volatiles were found. At one time the Moon outgassed prodigious amounts of water and hydrogen in volcanic fire fountains, but released similar amounts of volatile sulfur (or SO2), and presumably large amounts of carbon dioxide or monoxide, if theory is to be believed. So water on the Moon is associated with other gases. Astronomers have agreed for centuries that there is no firm evidence for “weather” on the Moon visible from Earth, and little evidence of thick atmosphere. [2] How would one detect the Moon’s atmosphere from Earth? An obvious means is atmospheric refraction. As you watch the Sun set, its image is displaced by Earth’s atmospheric refraction at the horizon from the position it would have if there were no atmosphere, by roughly 0.6 degree (a bit more than the Sun’s angular diameter). On the Moon, any atmosphere would cause an analogous effect for a star passing behind the Moon during an occultation (multiplied by two since the light travels both into and out of the lunar atmosphere). -

Forensics and Microscopy in Authenticating Works of Art Peter Paul Biro
Forensics and Microscopy in Authenticating Works of Art Peter Paul Biro 4 ISSUE 1 MARCH 2006 Fingerprints have been used around the world for identifying individuals since 1908. The availability of such evidence on works of art has been overlooked until the authentication of a Turner canvas in 1985. Since that case, a new methodology has been developed and the new discipline of forensic authentication was born. More recently, the concept of fingerprinting encompasses not only the marks left behind by our fingers but also the materials and working methods, widening the available ways to identify an artist. This innovative forensic approach has helped resolve equivocation and identify numerous important works of art as well as opening up a new field of research in art. bout 20 years ago, a client hang it as a demonstration. We gave walked into our Montreal in and a deal was struck. Some Aconservation laboratory with months later, a small area of the a large canvas he wanted cleaned and painting was tested to see how it restored. On first glance the painting behaved. After removing a small area seemed heavily overpainted and of overpainting on the sky we were recently so. The client shook his awestruck at the beauty of the head at the estimate for cleaning it, original surface coming to light. and said that it was not worth the Excitement grew and considerable cost as it was a wreck anyway. He effort was put into removing the asked whether our company would heavy coat of paint hiding the original buy the painting - to which he was surface. -

Ceramics Monthly (ISSN 0009-0329) Is Published Monthly Except July and August by Professional Publications, Inc.—S
William C. Hunt........................................ Editor Barbara Tipton...................... Associate Editor Robert L. Creager........................ Art Director Ruth C. Butler.............................. Copy Editor Valentina Rojo....................... Editorial Assistant Mary Rushley............... Circulation Manager Connie Belcher .... Advertising Manager Spencer L. Davis.............................. Publisher Editorial, Advertising and Circulation Offices 1609 Northwest Boulevard, Box 12448, Columbus, Ohio 43212 (614) 488-8236 Ceramics Monthly (ISSN 0009-0329) is published monthly except July and August by Professional Publications, Inc.—S. L. Davis, Pres.; P. S. Emery, Sec.: 1609 North west Blvd., Columbus, Ohio 43212. Second class postage paid at Columbus, Ohio. Subscription Rates:One year SI6, two years $30, three years $40. Add $5 per year for subscriptions outside the U.S.A. Change of Address:Please give us four weeks advance notice. Send both the magazine wrapper label and your new address to Ceramics Monthly, Circulation Office, Box 12448, Columbus, Ohio 43212. Contributors: Manuscripts, photographs, color separations, color transparencies (in cluding 35mm slides), graphic illustrations, texts and news releases dealing with ceramic art are welcome and will be considered for publication. A booklet describing procedures for the preparation and submission of a man uscript is available upon request. Send man uscripts and correspondence about them to The Editor, Ceramics Monthly, Box 12448, Columbus, Ohio 43212. Indexing:Articles in each issue of Ceramics Monthly are indexed in the Art Index. A 20-year subject index (1953-1972) covering Ceramics Monthly feature articles, Sugges tions and Questions columns is available for $1.50, postpaid from the Ceramics Monthly Book Department, Box 12448, Columbus, Ohio 43212. Additionally, each year’s arti cles are indexed in the December issue. -

Curatorial Care of Easel Paintings
Appendix L: Curatorial Care of Easel Paintings Page A. Overview................................................................................................................................... L:1 What information will I find in this appendix?.............................................................................. L:1 Why is it important to practice preventive conservation with paintings?...................................... L:1 How do I learn about preventive conservation? .......................................................................... L:1 Where can I find the latest information on care of these types of materials? .............................. L:1 B. The Nature of Canvas and Panel Paintings............................................................................ L:2 What are the structural layers of a painting? .............................................................................. L:2 What are the differences between canvas and panel paintings?................................................. L:3 What are the parts of a painting's image layer?.......................................................................... L:4 C. Factors that Contribute to a Painting's Deterioration............................................................ L:5 What agents of deterioration affect paintings?............................................................................ L:5 How do paint films change over time?........................................................................................ L:5 Which agents -

Fictive Strategies
CONTENT 1 CONTENT 2 - 3 HOLLY ROBERTS - ABOUT 4 HOLLY ROBERTS - ARTIST STATEMENT 5 SUZANNE SBARGE - ABOUT 6 SUZANNE SBARGE - ARTIST STATEMENT 7 EXHIBITION STATEMENT 8 - 9 EDUCATIONAL ACTIVITY 10 - 12 BEHIND THE SCENES 13 - 17 WORKS IN SHOW 18 THANK YOU PAGE 1 ABOUT THE ARTIST HOLLY ROBERTS Holly Roberts’ first national exposure c a m e i n 1 9 8 9 with the publication of the monograph, "Holly Roberts", from the Untitled Series published by the Friends of Photography. Although her work has always been based on the photograph, it was the inclusion of paint that made it so distinct. As David Featherstone says in his introduction, “Roberts is a painter, yet it is the photograph underlying the paint, even when it can scarcely be seen, that gives the work its intriguing, mysterious power. Drawing from the iconography of primitive art, particularly that of the Native American, Mexican and Hispanic cultures of the Southwest, where she lives, she creates paintings that address a broad range of human emotions. While it is Roberts’ evolving interaction with the photograph that takes her to her finished work, it is the existence of the underlying photographic image— even when it is obscured by paint— that gives the work its powerful qualities and sets up the emotional challenge for the viewer.” PAGE 2 ABOUT THE ARTIST HOLLY ROBERTS Her work has continued to evolve, but she has reversed her original process of heavily overpainting the black and white silver print. She now works on top of a painted surface, developing a narrative scene with collaged photographic elements. -

Chapter 8: Conservation Treatment
Chapter 8: Conservation Treatment Page A. Overview ...................................................................................................................................................... 8:1 What is preservation and how is it accomplished? ......................................................................... 8:2 What is conservation treatment? .................................................................................................... 8:2 What is stabilization? ...................................................................................................................... 8:3 What is restoration? ........................................................................................................................ 8:3 Why use reproductions? ................................................................................................................. 8:4 Why should treatments be reversible? ............................................................................................ 8:4 What NPS guidance is available to help me make decisions about conservation treatment? ....... 8:4 When do I need a conservator? ...................................................................................................... 8:4 B. Factors to Consider Before Conservation Treatment ................................................................ 8:5 How will I know what conservation treatment is appropriate? ........................................................ 8:5 What guidelines should I follow when considering -

Chemical Building Products
About Nordic Ecolabelled Chemical building products Version 2.17 Background to ecolabelling 23 februari 2021 Content 1 Summary 3 2 Basic facts about the criteria 3 3 About the criteria review/revision 8 4 Justification of the requirements 9 4.1 Relevance, potential and steerability 10 4.2 General environmental requirements 15 4.3 Volatile organic and aromatic compounds (VOC, VAH and TVOC) 49 4.4 Quality requirements 58 4.5 Requirements concerning packaging, labelling, consumer information and recycling 69 4.6 Quality management, regulatory requirements and marketing 71 5 Changes compared to previous version 74 6 New criteria 77 7 References 78 Terms and definitions 82 Appendix 1 Other labels Appendix 2 RPS, MECO table and requirement distribution 097 Chemical building products, version 2.17, 23 February 2021 This document is a translation of an original in Swedish. In case of dispute, the original document should be taken as authoritative. Addresses In 1989, the Nordic Council of Ministers decided to introduce a voluntary official ecolabel, the Nordic Swan Ecolabel. These organisations/companies operate the Nordic ecolabelling system on behalf of their own country’s government. For more information, see the websites: Denmark Finland Iceland Ecolabelling Denmark Ecolabelling Finland Ecolabelling Iceland Danish Standards Foundation Urho Kekkosen katu 4-6 E Umhverfisstofnun Göteborg Plads 1, DK-2150 Nordhavn FI-00100 HELSINKI Suðurlandsbraut 24 Fischersgade 56, DK-9670 Løgstør Tel +358 9 61 22 50 00 IS-108 REYKJAVIK Tel: +45 72 300 450 [email protected] Tel: +354 5 91 20 00 [email protected] www.ecolabel.fi [email protected] www.ecolabel.dk www.svanurinn.is Norway Sweden Ecolabelling Norway Ecolabelling Sweden Henrik Ibsens gate 20 Box 38114 NO-0255 OSLO SE-100 64 STOCKHOLM This document may only be copied in its Tel: +47 24 14 46 00 Tel: +46 8 55 55 24 00 entirety and without any type of change. -
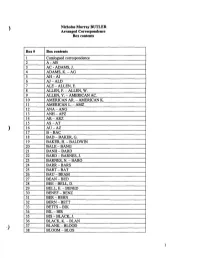
Nicholas Murray BUTLER Arranged Correspondence Box Contents Box
Nicholas Murray BUTLER Arranged Correspondence Box contents Box# Box contents 1 Catalogued correspondence 2 A-AB 3 AC - ADAMS, J. 4 ADAMS, K.-AG 5 AH-AI 6 AJ-ALD 7 ALE-ALLEN, E. 8 ALLEN, F.-ALLEN, W. 9 ALLEN, Y. - AMERICAN AC. 10 AMERICAN AR. - AMERICAN K. 11 AMERICAN L.-AMZ 12 ANA-ANG 13 ANH-APZ 14 AR-ARZ 15 AS-AT 16 AU-AZ 17 B-BAC 18 BAD-BAKER, G. 19 BAKER, H. - BALDWIN 20 BALE-BANG 21 BANH-BARD 22 BARD-BARNES, J. 23 BARNES, N.-BARO 24 BARR-BARS 25 BART-BAT 26 BAU-BEAM 27 BEAN-BED 28 BEE-BELL, D. 29 BELL,E.-BENED 30 BENEF-BENZ 31 BER-BERN 32 BERN-BETT 33 BETTS-BIK 34 BIL-BIR 35 BIS-BLACK, J. 36 BLACK, K.-BLAN 37 BLANK-BLOOD 38 BLOOM-BLOS 39 BLOU-BOD 40 BOE-BOL 41 BON-BOOK 42 BOOK-BOOT 43 BOR-BOT 44 BOU-BOWEN 45 BOWER-BOYD 46 BOYER-BRAL 47 BRAM-BREG 48 BREH-BRIC 49 BRID - BRIT 50 BRIT-BRO 51 BROG-BROOKS 52 BROOKS-BROWN 53 BROWN 54 BROWN-BROWNE 55 BROWNE -BRYA 56 BRYC - BUD 57 BUE-BURD 58 BURE-BURL 59 BURL-BURR 60 BURS-BUTC 61 BUTLER, A. - S. 62 BUTLER, W.-BYZ 63 C-CAI 64 CAL-CAMPA 65 CAMP - CANFIELD, JAMES H. (-1904) 66 CANFIELD, JAMES H. (1905-1910) - CANT 67 CAP-CARNA 68 CARNEGIE (1) 69 CARNEGIE (2) ENDOWMENT 70 CARN-CARR 71 CAR-CASTLE 72 CAT-CATH 73 CATL-CE 74 CH-CHAMB 75 CHAMC - CHAP 76 CHAR-CHEP 77 CHER-CHILD, K. -

Estate & Collectible Auction 11/22 14% Buyers Premium 717 S Third
Estate & Collectible Auction 11/22 14% Buyers Premium 717 S Third St Renton, WA (425) 235-6345 SILENT AUCTIONS Lots #1000-1114 Ends 7:00PM Lots #2000-2072 Ends 7:30PM Lots #3000-3072 Ends 8:00PM 20 Mink Stole 21 Group Ethnic Clothing Lot Description 22 Vintage Fur Coat & Stole 23 Fine Silk Ethnic Fabric 1 Southwest Upholstered Office Armchair 24 Chinese Silver Thread Embroidered Skirt 2 Ben Owen NC Pottery Jardinière 25 Patchwork Quilt 3 Shelf NC Blue Pottery 26 2 Framed Prints - David Lee & R.C. Gorman 4 (3) North Carolina Pottery Bean Pots 27 9ea Plastic Sleeves with Collectible Prints 5 Set (4) Ben Owen NC Pottery Crocks 28 Scottish Guard Framed Bullion Patch with Lids Display 6 2 Shelves Ben Owen NC Brown Pottery 29 19th Century Cherry Wood Cradle 7 Pair Long Scottish Guard Prints 30 Gold Metal Shoe Polish Cardboard Sign 8 Pair Walnut Mid Century Dining Chairs 31 Robert Werling Framed NW Photograph 8A Large Model Airplane Hanging 32 Vintage HO Toy Train Set 9 2ea Framed Lacework Panels 33 Painted Old Tavern Table 10 5ea Green Metal Vertical Document 34 Antique Wood Armchair Files from New Haven County Superior 35 Box Cloisonné Vases Court 36 Chinese Brass Bowl with Eggs 11 Set Leatherbound Catholic 37 Maple Lamp Table Encyclopedias 15 Volumes 1912 38 Singer Featherweight Sewing Machine in 11A 24 Volume Robert L. Stevenson Book Case Set 39 Painted Green Primitive Pine Table 11B Top Shelf Volume Book Sets 40 Rush Seat Pine Footstool NC 11C Set Waverleys Novels Books 41 Mohair Covered Footstool 12 Antique Eastlake Oak Bookcase 42 Cherry