Geographic and Depth Distributions of Decapod Shrimps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -
W7192e19.Pdf
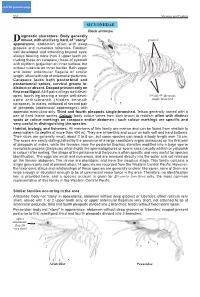
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Perspectives on Typhlatya (Crustacea, Decapoda)
Contributions to Zoology, 65 (2) 79-99 (1995) SPB Academic Publishing bv, Amsterdam New perspectives on the evolution of the genus Typhlatya (Crustacea, Decapoda): first record of a cavernicolous atyid in the Iberian Peninsula, Typhlatya miravetensis n. sp. Sebastián Sanz & Dirk Platvoet 1 Unitat d'Ecologia, Facultat de Ciències Biologiques, Universitat de Valencia, E-46100 Burjassot, 2 Valencia, Spain; Institutefor Systematics and Population Biology (Zoological Museum, Amsterdam), University of Amsterdam, P.O. Box 94766, 1090 GT Amsterdam, The Netherlands Keywords: Typhlatya, Decapoda, Spain, subterranean waters, systematics, zoogeography, vicariance, evolution, key to genus Abstract historia geológica de la zona y la distribución mundial del género, del grupo de géneros, y la familia. On several occasions, shrimps belonging to a new species ofthe genus Typhlatya were collected in a cave in the province of Castellón, Spain. This is the first record of the in the genus Introduction Iberian Peninsula. The species is described and the validity, dis- tribution, and zoogeography of the genus, as well as the status In 1993 and were on several of the discussed. 1994, shrimps caught genus Spelaeocaris, are Former models for the occasions in in the evolution of the genus Typhlatya and its genus group are re- a cave near Cabanes, province viewed, as well asthe system ofinner classification of the Atyidae of Castellón, eastern Spain. The specimens belong and its For the and evolution of biogeographical meaning. age the to genus Typhlatya Creaser, 1936, a genus the genus we developed a new model based on vicariance prin- with members known from the Galápagos Islands, ciples that involves further evolution of each species after the Ascension and the Caribbean of the ancestral This allows estimations Island, Bermuda, disruption range. -

CRUSTACEA Zooplankton (PELAGIC ADULTS) Sheet 112 ORDER: DECAPODA V
CONSEIL PERMANENT INTERNATIONAL POUR L’EXPLORATION DE LA MER CRUSTACEA Zooplankton (PELAGIC ADULTS) Sheet 112 ORDER: DECAPODA V. CARIDEA Families : Pasiphaeidae, Oplophoridae, Hippolytidae and Pandalidae (BY A. L. RICE) 1967 https://doi.org/10.17895/ices.pub.4953 Area considered -That part of the Atlantic to the north-east of a line joining Cape Farewell in Greenland and Cape St.Vincent in Portugal, including the Norwegian, Barents, North and Baltic Seas. 2 2 lb 4a Figure 1. Purupusiphue sulcutifrons Smith. (a) lateral view (after KEMP).(b) mandible (after SMITH).- Figure 2. Pusiphaeu sivudo (Risso). Tip of telson. - Figure 3. Pm$hueu multidentutu Esmark. (a) lateral view (after KEMP).(b) immovable finger of second leg. (c) basis and ischium of second leg. - Figure 4. Pusiphueu turdu Kreyer. (a) lateral view (after KEMP).(b) tip of telson. (c) basis and ischium of second leg. DECAPODA CARIDEA Anterior three pairs of thoracic limbs differentiated from the posterior five pairs as maxillipeds. Pleura of the second abdominal segment over- lapping those of the first and third segment. No chelae on the third legs. This definition distinguishes the Caridea from the Penaeidea, which have the pleura of the second abdominal segment not overlapping those of the first segment and also have chelate third legs, and from the Euphausiacea in which none of the thoracic limbs are modified as maxil- lipeds. (Several of the species dealt with in this sheet probably spend a good deal of their time as adults on or close to the sea bottom and make only occasional mid-water excursions. Some of the species are large and quite powerful swimmers and should perhaps be considered as nektonic rather than planktonic; they are included since they are often taken in large or high speed plankton samplers). -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

Redalyc.On Some Rare Oplophoridae (Caridea, Decapoda) from the South Mid-Atlantic Ridge
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Cardoso, Irene On some rare Oplophoridae (Caridea, Decapoda) from the South Mid-Atlantic Ridge Latin American Journal of Aquatic Research, vol. 41, núm. 2, abril, 2013, pp. 209-216 Pontificia Universidad Católica de Valparaíso Valparaiso, Chile Available in: http://www.redalyc.org/articulo.oa?id=175026114001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Lat. Am. J. Aquat. Res., 41(2): 209-216, 2013 Oplophoridae from south Mid-Atlantic Ridge 209 “Proceedings of the 3rd Brazilian Congress of Marine Biology” A.C. Marques, L.V.C. Lotufo, P.C. Paiva, P.T.C. Chaves & S.N. Leitão (Guest Editors) DOI: 10.3856/vol41-issue2-fulltext-1 Research Article On some rare Oplophoridae (Caridea, Decapoda) from the South Mid-Atlantic Ridge Irene Cardoso1 1Setor de Carcinologia, Museu Nacional, Quinta da Boa Vista São Cristóvão, Rio de Janeiro, 20940-040, Brazil ABSTRACT. The Mid-Atlantic Ridge (MAR) divides the Atlantic Ocean longitudinally into two halves, each with a series of major basins delimited by secondary, more or less transverse ridges. Recent biological investigations in this area were carried out within the framework of the international project Mar-Eco (Patterns and Processes of the Ecosystems of the Mid-Atlantic Ridge). In 2009 (from October, 25 to November, 29) 12 benthic sampling events were conducted on the R/V Akademik Ioffe, during the first oceanographic cruise of South Atlantic Mar-Eco. -

Crustacea: Decapoda) Can Penetrate the Abyss: a New Species of Lebbeus from the Sea of Okhotsk, Representing the Deepest Record of the Family
European Journal of Taxonomy 604: 1–35 ISSN 2118-9773 https://doi.org/10.5852/ejt.2020.604 www.europeanjournaloftaxonomy.eu 2020 · Marin I. This work is licensed under a Creative Commons Attribution Licence (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:7F2F71AA-4282-477C-9D6A-4C5FB417259D Thoridae (Crustacea: Decapoda) can penetrate the Abyss: a new species of Lebbeus from the Sea of Okhotsk, representing the deepest record of the family Ivan MARIN A.N. Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia. Email: [email protected], [email protected] urn:lsid:zoobank.org:author:B26ADAA5-5DBE-42B3-9784-3BC362540034 Abstract. Lebbeus sokhobio sp. nov. is described from abyssal depths (3303−3366 m) in the Kuril Basin of the Sea of Okhotsk. The related congeners are deep-water dwellers with a very distant distribution and very similar morphology. The new species is separated by minor morphological features, such as the armature of the rostrum and telson, meral spinulation of ambulatory pereiopods and the shape of the pleonal pleurae. This species is the deepest dwelling representative of the genus Lebbeus and the family Thoridae. A list of records of caridean shrimps recorded from abyssal depths below 3000 m is given. Keywords. Diversity, Caridea, barcoding, SokhoBio 2015, NW Pacifi c. Marin I. 2020. Thoridae (Crustacea: Decapoda) can penetrate the Abyss: a new species of Lebbeus from the Sea of Okhotsk, representing the deepest record of the family. European Journal of Taxonomy 604: 1–35. https://doi.org/10.5852/ejt.2020.604 Introduction The fauna of benthic caridean shrimps (Crustacea: Decapoda: Caridea) living at depths of more than 3000 m is poorly known due to the technical diffi culties of sampling. -

Distribution of Acanthephyra Brevicarinata Hanamura, 1984 and A
Zootaxa 3765 (6): 593–599 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3765.6.7 http://zoobank.org/urn:lsid:zoobank.org:pub:A2D2AF67-F55F-452D-842C-8623B3BC412E Distribution of Acanthephyra brevicarinata Hanamura, 1984 and A. brevirostris Smith, 1885 (Crustacea: Decapoda: Caridea: Acanthephyridae), in Pacific Mexico MICHEL E. HENDRICKX1,3 & DANIELA RÍOS-ELÓSEGUI2 1Laboratorio de Invertebrados Bentónicos, Unidad Académica Mazatlán Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, P.O. Box 811, Mazatlán Sinaloa 82000, Mexico. 2Posgrado en Ciencias del Mar y Limnología, Unidad Académica Mazatlán Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, P.O. Box 811, Mazatlán Sinaloa 82000, Mexico. E-mail: [email protected] 3Corresponding author. E-mail: [email protected] Abstract Two species of the Acanthephyridae, Acanthephyra brevicarinata Hanamura, 1984, and A. brevirostris Smith, 1885, are reported for the Pacific coast of Mexico. The number of known localities for A. brevicarinata, a species endemic to the eastern Pacific, is increased from 24 to 70 and the number of specimens on records from 160 to 363. New distribution limits are provided for this species, from 25°02’N; 112°54’W to 16°58’N; 100°55’W, including the central and northern Gulf of California from 28°01’N; 112°17’W southwards. Based on previous information related to its capture and the mor- phology of its first larval stage, A. brevicarinata is considered to be part of the nektobenthic fauna. -

THE OPLOPHORID and PASIPHAEID SHRIMP from OFF the OREGON COAST Redacted for Privacy Abstract Approved (Major Professor)
AN ABSTRACT OF THE THESIS OF Carl Albert Forss for the Ph. D. in Zoology (Name) (Degree) (Major) 1q1:1) Date thesis is presented l k` -} 1.-(2.t 1 Title THE OPLOPHORID AND PASIPHAEID SHRIMP FROM OFF THE OREGON COAST Redacted for Privacy Abstract approved (Major Professor) The 13 species of shrimps studied for this thesis were collected off the Oregon coast. The family Oplophoridae is well represented in this area. Five of the seven known genera were identified. Hymenodora frontalis, H. glacialis, and H. gracilis were described and further differentiating characters were illustrated. Other mem- bers of the family Oplophoridae were illustrated and described as follows: Acanthephyra curtirostris, Meningodora mollis, Notostomus japonicus, Systellaspis braueri and S. cristata. Species of the family Pasiphaeidae, although less well repre- sented, were identified. The presence or absence of the mandibular palp in these two species, Parapasiphaë sulcatifrons and P. cristata, found to be of little systematic value in separating them. Specimens with a carapace length of 7- 10mm lack a mandibular palp; one with a carapace length of 17 -25mm had a two -segmented palp. Pasiphaea pacifica, P. magna, and P. chacei were identified and illustrated. The taxonomy, geographical distribution, color and luminescence are discussed. THE OPLOPHORID AND PASIPHAEID SHRIMP FROM OFF THE OREGON COAST by CARL ALBERT FORSS A THESIS submitted to OREGON STATE UNIVERSITY in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY June 1966 APPROVED: Redacted for Privacy Professor of Zoology In Charge of Major Redacted for Privacy Chairman of Zoology Department Redacted for Privacy Dean of Graduate School Date thesis is presented ¿,C.,C-( .u%C ; . -

Zootaxa, Deep-Sea Oplophoridae (Crustacea Caridea)
ZOOTAXA 1031 Deep-sea Oplophoridae (Crustacea Caridea) from the southwestern Brazil IRENE CARDOSO & PAULO YOUNG Magnolia Press Auckland, New Zealand IRENE CARDOSO & PAULO YOUNG Deep-sea Oplophoridae (Crustacea Caridea) from the southwestern Brazil (Zootaxa 1031) 76 pp.; 30 cm. 8 Aug. 2005 ISBN 1-877407-24-0 (paperback) ISBN 1-877407-25-9 (Online edition) FIRST PUBLISHED IN 2005 BY Magnolia Press P.O. Box 41383 Auckland 1030 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2005 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 1031: 1–76 (2005) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1031 Copyright © 2005 Magnolia Press ISSN 1175-5334 (online edition) Deep-sea Oplophoridae (Crustacea Caridea) from the southwestern Brazil IRENE CARDOSO & PAULO YOUNG Museu Nacional / UFRJ, Quinta da Boa Vista, 20940-040 - Rio de Janeiro — RJ, Brazil email: [email protected] TABLE OF CONTENTS ABSTRACT . 3 INTRODUCTION . 4 MATERIAL AND METHODS . 7 SYSTEMATICS . 7 Family Oplophoridae Dana, 1852 . 7 Genus Acanthephyra Milne-Edwadrs, 1881 . 7 Acanthephyra acutifrons Bate, 1888 . 8 Acanthephyra eximia Smith, 1884 . 14 Acanthephyra quadrispinosa Kemp, 1939 . 21 Acanthephyra stylorostratis (Bate, 1888) . -

On Some Rare Oplophoridae (Caridea, Decapoda) from the South Mid-Atlantic Ridge
Lat. Am. J. Aquat. Res., 41(2): 209-216, 2013 Oplophoridae from south Mid-Atlantic Ridge 209 “Proceedings of the 3rd Brazilian Congress of Marine Biology” A.C. Marques, L.V.C. Lotufo, P.C. Paiva, P.T.C. Chaves & S.N. Leitão (Guest Editors) DOI: 10.3856/vol41-issue2-fulltext-1 Research Article On some rare Oplophoridae (Caridea, Decapoda) from the South Mid-Atlantic Ridge Irene Cardoso1 1Setor de Carcinologia, Museu Nacional, Quinta da Boa Vista São Cristóvão, Rio de Janeiro, 20940-040, Brazil ABSTRACT. The Mid-Atlantic Ridge (MAR) divides the Atlantic Ocean longitudinally into two halves, each with a series of major basins delimited by secondary, more or less transverse ridges. Recent biological investigations in this area were carried out within the framework of the international project Mar-Eco (Patterns and Processes of the Ecosystems of the Mid-Atlantic Ridge). In 2009 (from October, 25 to November, 29) 12 benthic sampling events were conducted on the R/V Akademik Ioffe, during the first oceanographic cruise of South Atlantic Mar-Eco. As a result we report some rare Oplophoridae species collected during the cruise. This family includes 73 species occurring strictly on the meso- and bathypelagic zones of the oceans. Five Oplophoridae species were sampled: Acanthephyra acanthitelsonis Bate, 1888; A. quadrispinosa Kemp, 1939; Heterogenys monnioti Crosnier, 1987; Hymenodora glacialis (Buchholz, 1874) and Kemphyra corallina (A. Milne-Edwards, 1883). Among these, H. monnioti and K. corallina are considered extremely rare, both with very few records. Of the sampled species, only A. quadrispinosa and H. glacialis were previously recorded to southwestern Atlantic, so the Oplophoridae fauna of the South MAR seems more related with the fauna from the eastern Atlantic and Indian oceans. -

An Overview of the Decapoda with Glossary and References
January 2011 Christina Ball Royal BC Museum An Overview of the Decapoda With Glossary and References The arthropods (meaning jointed leg) are a phylum that includes, among others, the insects, spiders, horseshoe crabs and crustaceans. A few of the traits that arthropods are characterized by are; their jointed legs, a hard exoskeleton made of chitin and growth by the process of ecdysis (molting). The Crustacea are a group nested within the Arthropoda which includes the shrimp, crabs, krill, barnacles, beach hoppers and many others. The members of this group present a wide range of morphology and life history, but they do have some unifying characteristics. They are the only group of arthropods that have two pairs of antenna. The decapods (meaning ten-legged) are a group within the Crustacea and are the topic of this key. The decapods are primarily characterized by a well developed carapace and ten pereopods (walking legs). The higher-level taxonomic groups within the Decapoda are the Dendrobranchiata, Anomura, Brachyura, Caridea, Astacidea, Axiidea, Gebiidea, Palinura and Stenopodidea. However, two of these groups, the Palinura (spiny lobsters) and the Stenopodidea (coral shrimps), do not occur in British Columbia and are not dealt with in this key. The remaining groups covered by this key include the crabs, hermit crabs, shrimp, prawns, lobsters, crayfish, mud shrimp, ghost shrimp and others. Arthropoda Crustacea Decapoda Dendrobranchiata – Prawns Caridea – Shrimp Astacidea – True lobsters and crayfish Thalassinidea - This group has recently