Contributions to Zoology, 72 (4) 195-209 (2003)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caudata: Hynobiidae): Heterochronies and Reductions
65 (1): 117 – 130 © Senckenberg Gesellschaft für Naturforschung, 2015. 4.5.2015 Development of the bony skeleton in the Taiwan salamander, Hynobius formosanus Maki, 1922 (Caudata: Hynobiidae): Heterochronies and reductions Anna B. Vassilieva 1 *, June-Shiang Lai 2, Shang-Fang Yang 2, Yu-Hao Chang 1 & Nikolay A. Poyarkov, Jr. 1 1 Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory, GSP-1, Moscow 119991, Russia — 2 Department of Life Science, National Taiwan Normal University, 88, Sec. 4 Tingchou Rd., Taipei 11677, Taiwan, R.O.C. — *Cor- responding author; vassil.anna(at)gmail.com Accepted 19.ii.2015. Published online at www.senckenberg.de / vertebrate-zoology on 4.v.2015. Abstract The development of the bony skeleton in a partially embryonized lotic-breeding salamander Hynobius formosanus is studied using the ontogenetic series from late embryos to postmetamorphic juveniles and adult specimen. Early stages of skull development in this spe- cies are compared with the early cranial ontogeny in two non-embryonized lentic-breeding species H. lichenatus and H. nigrescens. The obtained results show that skeletal development distinguishes H. formosanus from other hynobiids by a set of important features: 1) the reduction of provisory ossifications (complete absence of palatine and reduced state of coronoid), 2) alteration of a typical sequence of ossification appearance, namely, the delayed formation of vomer and coronoid, and 3) the absence of a separate ossification center of a lacrimal and formation of a single prefrontolacrimal. These unique osteological characters in H. formosanus are admittedly connected with specific traits of its life history, including partial embryonization, endogenous feeding until the end of metamorphosis and relatively short larval period. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

EFFECTS of LATITUDE, SEASON, ELEVATION, and MICROHABITAT on FIELD BODY TEMPERATURES of NEOTROPICAL and TEMPERATE ZONE Salamandersl
Ecology, 63(6), 1982, pp. 1657-1664 © 1982 by the Ecological Society of America EFFECTS OF LATITUDE, SEASON, ELEVATION, AND MICROHABITAT ON FIELD BODY TEMPERATURES OF NEOTROPICAL AND TEMPERATE ZONE SALAMANDERSl MARTIN E. FEDER Department ofAnatomy and Committee on Evolutionary Biology, The University of Chicago, Chicago, Illinois 60637 USA, and Museum of Vertebrate Zoology, University of California, Berkeley, California 94720 USA AND JAMES F. LYNCH Chesapeake Bay Center for Environmental Studies, Smithsonian Institution, P.O. Box 28, Edgewater, Maryland 21037 USA, and Museum of Vertebrate Zoology, University of California, Berkeley, California 94720 USA Abstract. We analyzed field body temperatures of neotropical salamanders (Feder et al. 1982b) to examine existing generalizations about salamander thermal ecology, which have been based almost entirely upon data for temperate zone species. Our findings can be summarized as follows: 1) Behavioral thermoregulation in the field is evidently uncommon among the vast majority of tropical and temperate salamander species. Body temperatures of tropical salamanders closely parallel seasonal and altitudinal changes in the thermal environment. 2) Body temperatures of salamanders show a complex relationship with latitude. Temperate zone species experience lower minimum temperatures than neotropical salamanders, but there are no consistent latitudinal trends in maximum body temperatures. Tropical plethodontids and ambysto matids show similar rates of decline in mean body temperature with increasing elevation, but am bystomatid temperatures are significantly warmer than plethodontid temperatures at the same ele vation. 3) Variation in body temperature is greater seasonally for temperate salamanders than tropical salamanders. At a given time or locality, however, variation in field body temperature among members of a population is similar for tropical and temperate salamanders. -

Across Watersheds in the Klamath Mountains
Diversity 2013, 5, 657-679; doi:10.3390/d5030657 OPEN ACCESS diversity ISSN 1424-2818 www.mdpi.com/journal/diversity Article Genetic Diversity of Black Salamanders (Aneides flavipunctatus) across Watersheds in the Klamath Mountains Sean B. Reilly 1,*, Mitchell F. Mulks 2, Jason M. Reilly 3, W. Bryan Jennings 4, and David B. Wake 1 1 Museum of Vertebrate Zoology and Department of Integrative Biology, University of California, 3101 Valley Life Sciences Building, Berkeley, CA 94720-3160, USA; E-Mail: [email protected] 2 Department of Ecology and Evolutionary Biology, University of California, 1156 High Street, Santa Cruz, CA 95064, USA; E-Mail: [email protected] 3 Bureau of Land Management, Medford Interagency Office, Medford, OR 97504, USA; E-Mail: [email protected] 4 Museu Nacional, Departamento de Vertebrados, Universidade Federal Do Rio de Janeiro, Rio de Janeiro, RJ 20940-040, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-510-642-3567; Fax: +1-510-643-8238. Received: 31 May 2013; in revised form: 2 August 2013 / Accepted: 8 August 2013 / Published: 29 August 2013 Abstract: Here we characterize the genetic structure of Black Salamanders (Aneides flavipunctatus) in the Klamath Mountains of northwestern California and southwestern Oregon using mitochondrial and nuclear DNA sequences. We hypothesized that the Sacramento, Smith, Klamath, and Rogue River watersheds would represent distinct genetic populations based on prior ecological results, which suggest that Black Salamanders avoid high elevations such as the ridges that separate watersheds. Our mitochondrial results revealed two major lineages, one in the Sacramento River watershed, and another containing the Klamath, Smith, and Rogue River watersheds. -

Synchrotron Microtomography Applied to the Volumetric Analysis of Internal Structures of Thoropa Miliaris Tadpoles G
www.nature.com/scientificreports OPEN Synchrotron microtomography applied to the volumetric analysis of internal structures of Thoropa miliaris tadpoles G. Fidalgo1*, K. Paiva1, G. Mendes1, R. Barcellos1, G. Colaço2, G. Sena1, A. Pickler1, C. L. Mota1, G. Tromba3, L. P. Nogueira4, D. Braz5, H. R. Silva2, M. V. Colaço1 & R. C. Barroso1 Amphibians are models for studying applied ecological issues such as habitat loss, pollution, disease, and global climate change due to their sensitivity and vulnerability to changes in the environment. Developmental series of amphibians are informative about their biology, and X-ray based 3D reconstruction holds promise for quantifying morphological changes during growth—some with a direct impact on the possibility of an experimental investigation on several of the ecological topics listed above. However, 3D resolution and discrimination of their soft tissues have been difcult with traditional X-ray computed tomography, without time-consuming contrast staining. Tomographic data were initially performed (pre-processing and reconstruction) using the open- source software tool SYRMEP Tomo Project. Data processing and analysis of the reconstructed tomography volumes were conducted using the segmentation semi-automatic settings of the software Avizo Fire 8, which provide information about each investigated tissues, organs or bone elements. Hence, volumetric analyses were carried out to quantify the development of structures in diferent tadpole developmental stages. Our work shows that synchrotron X-ray microtomography using phase-contrast mode resolves the edges of the internal tissues (as well as overall tadpole morphology), facilitating the segmentation of the investigated tissues. Reconstruction algorithms and segmentation software played an important role in the qualitative and quantitative analysis of each target structure of the Thoropa miliaris tadpole at diferent stages of development, providing information on volume, shape and length. -

The Spemann Organizer Meets the Anterior‐
The Japanese Society of Developmental Biologists Develop. Growth Differ. (2015) 57, 218–231 doi: 10.1111/dgd.12200 Original Article The Spemann organizer meets the anterior-most neuroectoderm at the equator of early gastrulae in amphibian species Takanori Yanagi,1,2† Kenta Ito,1,2† Akiha Nishihara,1† Reika Minamino,1,2 Shoko Mori,1 Masayuki Sumida3 and Chikara Hashimoto1,2* 1JT Biohistory Research Hall, 1-1 Murasaki-cho, Takatsuki, Osaka 569-1125, 2Department of Biological Sciences, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, and 3Institute for Amphibian Biology, Hiroshima University, Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8526, Japan The dorsal blastopore lip (known as the Spemann organizer) is important for making the body plan in amphibian gastrulation. The organizer is believed to involute inward and migrate animally to make physical contact with the prospective head neuroectoderm at the blastocoel roof of mid- to late-gastrula. However, we found that this physical contact was already established at the equatorial region of very early gastrula in a wide variety of amphibian species. Here we propose a unified model of amphibian gastrulation movement. In the model, the organizer is present at the blastocoel roof of blastulae, moves vegetally to locate at the region that lies from the blastocoel floor to the dorsal lip at the onset of gastrulation. The organizer located at the blastocoel floor con- tributes to the anterior axial mesoderm including the prechordal plate, and the organizer at the dorsal lip ends up as the posterior axial mesoderm. During the early step of gastrulation, the anterior organizer moves to estab- lish the physical contact with the prospective neuroectoderm through the “subduction and zippering” move- ments. -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -

A Herpetological Survey of Dixie Caverns and Explore Park in Roanoke, Virginia and the Wehrle’S Salamander
A Herpetological Survey of Dixie Caverns and Explore Park in Roanoke, Virginia and the Wehrle’s Salamander Matthew Neff Department of Herpetology National Zoological Park Smithsonian Institution MRC 5507, Washington, DC 20013 Introduction The Virginia Herpetological Society (VHS) Dixie Caverns Survey was held at Dixie Caverns and Explore Park in Roanoke County, Virginia on 24 September 2016. According to legend, Dixie Caverns was discovered in 1920 by two young men after their dog Dixie fell through a hole that led to the caves. In honor of their dog’s discovery, they decided to name the caverns Dixie. One of those boys was Bill “Shorty” McDaniel who would later go on to work at the caverns for more than 50 years and was known fondly for his sometimes embellished stories (Berrier, 2014). In actuality, the presence of Dixie Caverns, according to The Roanoke Times, was known as early as 1860 and had been mapped in the early 1900’s (Berrier, 2014). Guided tours of the caverns began in 1923 and still occur today with about 30,000 people visiting annually (Berrier, 2014). Dixie Caverns is located in Roanoke County which is in the Valley and Ridge and Blue Ridge provinces (Mitchell, 1999). A key feature of the Valley and Ridge is karst topography with soluble rocks such as limestone which create caves and caverns when weathered (Tobey, 1985). Over millions of years the caverns were formed as water dissolved the limestone that created Catesbeiana 38(1):20-36 20 Dixie Caverns and Explore Park Survey holes and even larger passageways. Many of the rock formations in Dixie Caverns are made of calcite which was formed by dripping water that evaporated leaving behind tiny particles which eventually created stalactites (Berrier, 2014). -

Preliminary Study of Food Habits in the Japanese Clawed Salamander Larvae (Onychodactylus Japonicus) in a Mountain Brook of the Kiso River System
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kochi University Repository 黒潮圏科学(Kuroshio・Science),4−2,175−181,2011 Preliminary study of food habits in the Japanese clawed salamander larvae (Onychodactylus japonicus) in a mountain brook of the Kiso River system Teruhiko Takahara1,2*, Motomi Genkai-Kato1,3, Hitoshi Miyasaka1 and Yukihiro Kohmatsu1,2 1 Kiso Biological Station, Kyoto University (6388 Fukushima, Kiso, Nagano 397-0001) 2 Research Institute for Humanity and Nature (457-4 Motoyama, Kamigamo, Kita-ku, Kyoto 603-8047) 3 Graduate School of Kuroshio Science, Kochi University (2-5-1 Akebono-cho, Kochi 780-8520) Abstract To evaluate food habits of the Japanese clawed salamander larvae (Onychodactylus japonicus), we exam- ined stomach contents of 22 individuals collected from a natural mountain brook in a tributary of the Kuro- kawa River in Kiso Fukushima, Nagano Prefecture, central Japan. Their diet composition did not differ between fast and slow current conditions. The diet reflected the natural benthos communities of the brook, in which mayfly nymphs and caddisfly larvae accounted for 70–88%. The salamander larvae selectively foraged on caddisfly and fly larvae in the two current conditions. In the slow current condition, their prey preference was detected in the prey group including terrestrial invertebrates. Our results suggest that the prey items of O. japonicus larvae hinge primarily on the natural benthos communities. Key words: benthic animals, habitat traits, Manly’s Index, prey preference, terrestrial invertebrates Introduction regions ranging from a few hundred meters to over 2000 m above sea level (Goris and Maeda, 2004). -
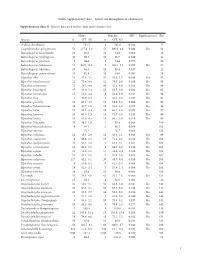
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Salamandrella Keyserlingii, Amphibia, Caudata) and the Cryptic Species S
Entomological Review, Vol. 85, Suppl. 2, 2005, pp. S240–S253. Translated from Zoologicheskii Zhurnal, Vol. 84, no. 11, 2005. Original Russian Text Copyright © 2005 by Berman, Derenko, Malyarchuk, Grzybowski, Kryukov, Miscicka-Sliwka. English Translation Copyright © 2005 by Pleiades Publishing, Inc. Intraspecific Genetic Differentiation of the Siberian Newt (Salamandrella keyserlingii, Amphibia, Caudata) and the Cryptic Species S. schrenckii from Southeastern Russia D. I. Berman*, M. V. Derenko*, B. A. Malyarchuk*, T. Grzybowski**, A. P. Kryukov***, and D. Miscicka-Sliwka** *Institute of Biological Problems of the North, Far East Division, Russian Academy of Sciences, Magadan, 685000 Russia e-mail: [email protected], [email protected] **Forensic Medicine Institute, Ludwik Rydygier Medical University, Bydgoszcz, 85-094 Poland ***Institute of Biology and Soil Science, Far East Division, Russian Academy of Sciences, Vladivostok, 690022 Russia Received March 16, 2005 Abstract—The nucleotide sequences of the mitochondrial cytochrome b gene in the Siberian newt Salaman- drella keyserlingii Dybowski 1870 from the populations of the Ural Mountains, Magadan oblast, Chukchi Pen- insula, Sakhalin Island, and Primorskii krai are analyzed. It is shown that in most populations studied (except for Primorskii krai), a low geographic variation in morphological characters corresponds to a low level of genetic variation (0.38% in the combined sample from the Magadan, Sakhalin, Chukchi, and Ural populations). Different scenarios for the origin of the genetically and morphologically homogeneous hyperpopulation are dis- cussed, taking into account the obvious lack of genetic exchange between the marginal populations of the range. They involve the rapid formation of the species range in the Holocene, which followed its gradual development in the Pleistocene; unidirectional stabilizing selection within the entire range; the maintenance of variation at a stable level by mixing of the population during the dispersal of the young and, possibly, by group fertilization. -

Salamander Species Listed As Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016
Salamander Species Listed as Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016 Effective January 28, 2016, both importation into the United States and interstate transportation between States, the District of Columbia, the Commonwealth of Puerto Rico, or any territory or possession of the United States of any live or dead specimen, including parts, of these 20 genera of salamanders are prohibited, except by permit for zoological, educational, medical, or scientific purposes (in accordance with permit conditions) or by Federal agencies without a permit solely for their own use. This action is necessary to protect the interests of wildlife and wildlife resources from the introduction, establishment, and spread of the chytrid fungus Batrachochytrium salamandrivorans into ecosystems of the United States. The listing includes all species in these 20 genera: Chioglossa, Cynops, Euproctus, Hydromantes, Hynobius, Ichthyosaura, Lissotriton, Neurergus, Notophthalmus, Onychodactylus, Paramesotriton, Plethodon, Pleurodeles, Salamandra, Salamandrella, Salamandrina, Siren, Taricha, Triturus, and Tylototriton The species are: (1) Chioglossa lusitanica (golden striped salamander). (2) Cynops chenggongensis (Chenggong fire-bellied newt). (3) Cynops cyanurus (blue-tailed fire-bellied newt). (4) Cynops ensicauda (sword-tailed newt). (5) Cynops fudingensis (Fuding fire-bellied newt). (6) Cynops glaucus (bluish grey newt, Huilan Rongyuan). (7) Cynops orientalis (Oriental fire belly newt, Oriental fire-bellied newt). (8) Cynops orphicus (no common name). (9) Cynops pyrrhogaster (Japanese newt, Japanese fire-bellied newt). (10) Cynops wolterstorffi (Kunming Lake newt). (11) Euproctus montanus (Corsican brook salamander). (12) Euproctus platycephalus (Sardinian brook salamander). (13) Hydromantes ambrosii (Ambrosi salamander). (14) Hydromantes brunus (limestone salamander). (15) Hydromantes flavus (Mount Albo cave salamander).