The Spemann Organizer Meets the Anterior‐
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Emerging Infectious Diseases on Amphibians: a Review of Experimental Studies
diversity Review Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies Andrew R. Blaustein 1,*, Jenny Urbina 2 ID , Paul W. Snyder 1, Emily Reynolds 2 ID , Trang Dang 1 ID , Jason T. Hoverman 3 ID , Barbara Han 4 ID , Deanna H. Olson 5 ID , Catherine Searle 6 ID and Natalie M. Hambalek 1 1 Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA; [email protected] (P.W.S.); [email protected] (T.D.); [email protected] (N.M.H.) 2 Environmental Sciences Graduate Program, Oregon State University, Corvallis, OR 97331, USA; [email protected] (J.U.); [email protected] (E.R.) 3 Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN 47907, USA; [email protected] 4 Cary Institute of Ecosystem Studies, Millbrook, New York, NY 12545, USA; [email protected] 5 US Forest Service, Pacific Northwest Research Station, Corvallis, OR 97331, USA; [email protected] 6 Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA; [email protected] * Correspondence [email protected]; Tel.: +1-541-737-5356 Received: 25 May 2018; Accepted: 27 July 2018; Published: 4 August 2018 Abstract: Numerous factors are contributing to the loss of biodiversity. These include complex effects of multiple abiotic and biotic stressors that may drive population losses. These losses are especially illustrated by amphibians, whose populations are declining worldwide. The causes of amphibian population declines are multifaceted and context-dependent. One major factor affecting amphibian populations is emerging infectious disease. Several pathogens and their associated diseases are especially significant contributors to amphibian population declines. -

Local Population Differentiation and Phylogenetic
Japanese Journal of Herpetology 17(3): 91-97., June 1998 (C)1998 by The HerpetologicalSociety of Japan Local Population Differentiation and Phylogenetic Relationships of Russian Brown Frog, Rana amurensis Inferred by Mitochon- drial Cytochrome b Gene Sequences (Amphibia, Ranidae) TOMOKO TANAKA-UENO, MASAFUMI MATSUI, TAKANORI SATO, SEN TAKENAKA AND OSAMU TAKENAKA Abstract: In order to assess local population differentiation and phylogenetic relation- ships of a Russian brown frog, Rana amurensis, the sequences of 587 base pairs of the mitochondrial cytochrome b genes are compared with seven species of Japanese and one species complex of Taiwanese brown frogs (R. pirica, R. ornativentris, R. dybowskii, R. japonica, R. okinavana, R. tagoi, R. tsushimensis, and the R. sauteri complex). Genetic differentiation between populations of R. amurensis from Sakhalin and the Maritime Territory was found to be minimal. The resultant phylogenetic tree indicates monophyly of brown frogs and earliest divergence of R. amurensis among all the brown frogs studied. For this reason, separation of R. amurensis from the R. japonica group is suggested, but separation of the R. sauteri complex as a distinct ge- nus or subgenus Pseudorana is not supported. Key words: Brown frog; Cytochrome b; Phylogeny; Pseudorana; Rana amurensis; Russia Rana amurensis Boulenger, 1886 occupies a ships of Japanese brown frogs have been report- very wide range, including western Siberia east ed by Nishioka et al. (1992) and Tanaka et al. to Sakhalin, northern and eastern Mongolia, (1996). In the present study, we first investigat- northeastern China and Korea, and regions ed the degree of genetic differentiation between north to bevond the Arctic Circle to 71°N populations of R, amurensis in Sakhalin and (Frost, 1985). -

Phylogenetic Relationships of Brown Frogs from Taiwan and Japan Assessed by Mitochondrial Cytochrome B Gene Sequences (Rana: Ranidae)
ZOOLOGICAL SCIENCE 15: 283–288 (1998) © 1998 Zoological Society of Japan Phylogenetic Relationships of Brown Frogs from Taiwan and Japan Assessed by Mitochondrial Cytochrome b Gene Sequences (Rana: Ranidae) Tomoko Tanaka-Ueno1*, Masafumi Matsui1, Szu-Lung Chen2, Osamu Takenaka3 and Hidetoshi Ota4 1Graduate School of Human and Environmental Studies, Kyoto University, Sakyo-ku, Kyoto 606-01, Japan 2Department of Zoology, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-01, Japan 3Primate Research Institute, Kyoto University, Inuyama, Aichi 484, Japan 4Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-01, Japan ABSTRACT—In order to assess phylogenetic relationships of Taiwanese brown frogs (Rana longicrus and the R. sauteri complex), the partial sequences (587 base pairs) of the mitochondrial cytochrome b genes were compared with six brown frogs from Japan (R. pirica, R. ornativentris, R. japonica, R. tagoi tagoi, R. tsushimensis, and R. okinavana). Resultant phylogenetic trees indicated a considerable genetic differentia- tion between R. longicrus and R. japonica in spite of their close morphological and ecological similarities. The R. sauteri complex includes two genetically distinct groups that are not consistent with current classifica- tion. One group including populations of Alishan (central Taiwan) and Sanyi (western Taiwan) seemed to be closest to R. tagoi and the presumptive common ancestor of these frogs is thought to have diverged very early. Another group including a population from Wulai (northern Taiwan) showed a sister relationship with R. tsushimensis and R. okinavana, both isolated on small islands of Japan. These Taiwanese and Japanese brown frogs as a whole form a monophyletic group, and separation of the R. -

Molekularno-Filogenetičke I Morfološke Značajke Zelenih Žaba (Rod Pelophylax) U Hrvatskoj
Molekularno-filogenetičke i morfološke značajke zelenih žaba (rod Pelophylax) u Hrvatskoj Schmidt, Bruno Master's thesis / Diplomski rad 2018 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: University of Zagreb, Faculty of Science / Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:217:811997 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-10-04 Repository / Repozitorij: Repository of Faculty of Science - University of Zagreb Sveučilište u Zagrebu Prirodoslovno – matematički fakultet Biološki odsjek Bruno Schmidt Molekularno–filogenetičke i morfološke značajke zelenih žaba (rod Pelophylax) u Hrvatskoj Diplomski rad Zagreb, 2018. Ovaj rad izrađen je na Zoologijskom zavodu Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu, pod vodstvom prof. dr. sc. Görana Klobučara i pomoćnim voditeljstvom dr. sc. Mišela Jelića. Rad je predan na ocjenu Biološkom odsjeku Prirodoslovnog-matematičkog fakulteta Sveučilišta u Zagrebu radi stjecanja zvanja magistra znanosti o okolišu. 2 Najljepše zahvaljujem mentoru dr. sc. Göranu Klobučaru na ukazanom povjerenju, stručnom vodstvu te vremenu i trudu kojeg je uložio. Najveća hvala dr. sc. Mišelu Jeliću koji me vodio kroz ovo istraživanje, rješavao sve probleme i prepreke, značajno unaprijedio moje znanje i pokazao mi kako se pravilno izvodi znanstveno istraživanje. Velika hvala svim starim i novim prijateljima koje stekao tijekom svog akademskog obrazovanja, koji su mi pružili potporu -

Download Download
HAMADRYAD Vol. 27. No. 2. August, 2003 Date of issue: 31 August, 2003 ISSN 0972-205X CONTENTS T. -M. LEONG,L.L.GRISMER &MUMPUNI. Preliminary checklists of the herpetofauna of the Anambas and Natuna Islands (South China Sea) ..................................................165–174 T.-M. LEONG & C-F. LIM. The tadpole of Rana miopus Boulenger, 1918 from Peninsular Malaysia ...............175–178 N. D. RATHNAYAKE,N.D.HERATH,K.K.HEWAMATHES &S.JAYALATH. The thermal behaviour, diurnal activity pattern and body temperature of Varanus salvator in central Sri Lanka .........................179–184 B. TRIPATHY,B.PANDAV &R.C.PANIGRAHY. Hatching success and orientation in Lepidochelys olivacea (Eschscholtz, 1829) at Rushikulya Rookery, Orissa, India ......................................185–192 L. QUYET &T.ZIEGLER. First record of the Chinese crocodile lizard from outside of China: report on a population of Shinisaurus crocodilurus Ahl, 1930 from north-eastern Vietnam ..................193–199 O. S. G. PAUWELS,V.MAMONEKENE,P.DUMONT,W.R.BRANCH,M.BURGER &S.LAVOUÉ. Diet records for Crocodylus cataphractus (Reptilia: Crocodylidae) at Lake Divangui, Ogooué-Maritime Province, south-western Gabon......................................................200–204 A. M. BAUER. On the status of the name Oligodon taeniolatus (Jerdon, 1853) and its long-ignored senior synonym and secondary homonym, Oligodon taeniolatus (Daudin, 1803) ........................205–213 W. P. MCCORD,O.S.G.PAUWELS,R.BOUR,F.CHÉROT,J.IVERSON,P.C.H.PRITCHARD,K.THIRAKHUPT, W. KITIMASAK &T.BUNDHITWONGRUT. Chitra burmanica sensu Jaruthanin, 2002 (Testudines: Trionychidae): an unavailable name ............................................................214–216 V. GIRI,A.M.BAUER &N.CHATURVEDI. Notes on the distribution, natural history and variation of Hemidactylus giganteus Stoliczka, 1871 ................................................217–221 V. WALLACH. -

Summary Report of Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 5
Summary Report of Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 5 Summary Report of Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 5 Prepared by: Amy J. Benson, Colette C. Jacono, Pam L. Fuller, Elizabeth R. McKercher, U.S. Geological Survey 7920 NW 71st Street Gainesville, Florida 32653 and Myriah M. Richerson Johnson Controls World Services, Inc. 7315 North Atlantic Avenue Cape Canaveral, FL 32920 Prepared for: U.S. Fish and Wildlife Service 4401 North Fairfax Drive Arlington, VA 22203 29 February 2004 Table of Contents Introduction ……………………………………………………………………………... ...1 Aquatic Macrophytes ………………………………………………………………….. ... 2 Submersed Plants ………...………………………………………………........... 7 Emergent Plants ………………………………………………………….......... 13 Floating Plants ………………………………………………………………..... 24 Fishes ...…………….…………………………………………………………………..... 29 Invertebrates…………………………………………………………………………...... 56 Mollusks …………………………………………………………………………. 57 Bivalves …………….………………………………………………........ 57 Gastropods ……………………………………………………………... 63 Nudibranchs ………………………………………………………......... 68 Crustaceans …………………………………………………………………..... 69 Amphipods …………………………………………………………….... 69 Cladocerans …………………………………………………………..... 70 Copepods ……………………………………………………………….. 71 Crabs …………………………………………………………………...... 72 Crayfish ………………………………………………………………….. 73 Isopods ………………………………………………………………...... 75 Shrimp ………………………………………………………………….... 75 Amphibians and Reptiles …………………………………………………………….. 76 Amphibians ……………………………………………………………….......... 81 Toads and Frogs -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -
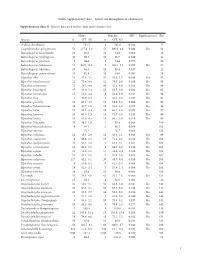
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

New Host Records for Lernaea Cyprinacea (Copepoda), a Parasite of Freshwater Fishes, with a Checklist of the Lernaeidae in Japan (1915-2007)
J. Grad. Sch. Biosp. Sci. Hiroshima Univ. (2007), 46:21~33 New Host Records for Lernaea cyprinacea (Copepoda), a Parasite of Freshwater Fishes, with a Checklist of the Lernaeidae in Japan (1915-2007) Kazuya Nagasawa, Akiko Inoue, Su Myat and Tetsuya Umino Graduate School of Biosphere Science, Hiroshima University 1-4-4 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8528, Japan Abstract The lernaeid copepod Lernaea cyprinacea Linnaeus, 1758, was found attached to three species of freshwater fishes, the barbell steed Hemibarbus labeo (Pallas) (Cyprinidae), the dark chub Zacco temminckii (Temminck and Schlegel) (Cyprinidae), and the Amur catfish Silurus asotus Linnaeus (Siluridae) from Hiroshima Prefecture in Japan. The findings from Hemibarbus labeo and Zacco temminckii represent new host records for L. cyprinacea, while Silurus asotus is a new host in Japan. Based on the literature published for 93 years from 1915 to 2007, a checklist of three species of lernaeid copepods (Lernaea cyprinacea, Lernaea parasiluri, Lamproglena chinensis) from Japan is given, including information on the synonym(s), host(s), site(s) of infection, and distribution. The checklist shows that in Japan L. cyprinacea has been reported from 33 or 34 species and subspecies of fishes belonging to 17 families in 10 orders and also from 2 species of amphibians from 2 families in 2 orders. Key words: Lamproglena chinensis; Lernaea cyprinacea; Lernaea parasiluri; Lernaeidae; parasites; new hosts INTRODUCTION The lernaeid copepod Lernaea cyprinacea Linnaeus, 1758, often called the anchor worm, is a parasite of freshwater fishes in various regions of the world (Kabata, 1979; Lester and Hayward, 2006). The anterior part of the body of metamorphosed adult female is embedded in the host tissue, whereas the remaining body protrudes in the water. -

No Evidence for the 'Rate-Of-Living' Theory Across the Tetrapod Tree of Life
Received: 23 June 2019 | Revised: 30 December 2019 | Accepted: 7 January 2020 DOI: 10.1111/geb.13069 RESEARCH PAPER No evidence for the ‘rate-of-living’ theory across the tetrapod tree of life Gavin Stark1 | Daniel Pincheira-Donoso2 | Shai Meiri1,3 1School of Zoology, Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel Abstract 2School of Biological Sciences, Queen’s Aim: The ‘rate-of-living’ theory predicts that life expectancy is a negative function of University Belfast, Belfast, United Kingdom the rates at which organisms metabolize. According to this theory, factors that accel- 3The Steinhardt Museum of Natural History, Tel Aviv University, Tel Aviv, Israel erate metabolic rates, such as high body temperature and active foraging, lead to organismic ‘wear-out’. This process reduces life span through an accumulation of bio- Correspondence Gavin Stark, School of Zoology, Faculty of chemical errors and the build-up of toxic metabolic by-products. Although the rate- Life Sciences, Tel Aviv University, Tel Aviv, of-living theory is a keystone underlying our understanding of life-history trade-offs, 6997801, Israel. Email: [email protected] its validity has been recently questioned. The rate-of-living theory has never been tested on a global scale in a phylogenetic framework, or across both endotherms and Editor: Richard Field ectotherms. Here, we test several of its fundamental predictions across the tetrapod tree of life. Location: Global. Time period: Present. Major taxa studied: Land vertebrates. Methods: Using a dataset spanning the life span data of 4,100 land vertebrate spe- cies (2,214 endotherms, 1,886 ectotherms), we performed the most comprehensive test to date of the fundamental predictions underlying the rate-of-living theory. -

Study on Conservation Ecology of Endangered Freshwater Animal
■ Introduction to Research 〓 Research Area : Vegetation Management Study on conservation of ecosystem based on weed vegetation and risk evaluation of invasive plants In all over the world, raising con- cern about declining ecosystem services by development and en- vironmental pollution. Weed vegetation supports the ecosystemic base while contact- ing with human activities. Therefore, we tackle improvement of environmental problem by un- derstanding and utilizing a func- tion of the weed vegetation. On the other hand, the disturbance of an eco- system caused by the invasive alien species is serious, too. We propose ecosystemic 〓 Asst. Prof. precise management NAKASHIMA Yoshitaka method by grasping sea- sonal variation in emer- gence and elucidating the influence to environ- ment of alien plants. 〓 Research Area : Applied Ecology Study on conservation ecology of endangered freshwater animal species Many native species of freshwater animals are endangered due to factors such as the negative effects of river or agricultural channel improvements and predation or competitive exclusion by invasive species. To conserve such en- dangered native freshwater animals, we have to clarify the basic ecology (e.g., reproductive behavior and habitat preference) and then develop effective con- servation methods. In our recent studies, we clarified the habitat preference of the endangered bitterling fish species (Rhodeus atremius suigensis; Fig. 1) in agricultural channels, and wintering site environment of the endangered Nagoya Daruma Pond Frog (Pelophylax porosus brevipodus) inhabiting pad- dy fields. Also, we have studied the effectiveness of restoration methods in Fig. 1 An endangered bitterling agricultural channels for freshwater fish conservation. fish (R. atremius suigensis). Study on ecology of invasive crayfish species and development of effective eradication methods Invasive freshwater animal species have a negative impact on native species. -

Current Knowledge of Ghrelin in Amphibians
2017, 64 (Suppl.), S15-S19 Current knowledge of ghrelin in amphibians Hiroyuki Kaiya1), Kenji Kangawa2) and Mikiya Miyazato1) 1) Department of Biochemistry, National Cerebral and Cardiovascular Center Research Institute, Suita 565-8565, Japan 2) National Cerebral and Cardiovascular Center Research Institute, Suita 565-8565, Japan Abstract. We are exploring physiological importance of the ghrelin system in vertebrates. This review summarizes current knowledge of the ghrelin system in amphibians. Our study on ghrelin precursor in various amphibians revealed that the third amino acid with acyl modification has changed to threonine (Thr-3) instead of serine (Ser-3) only in the genus, Rana. Functional analyses of the ghrelin receptor in three species of amphibians, Japanese fire belly newt, American bullfrog and Japanese tree frog revealed that ghrelin and GHS-R1a agonists increase intracellular Ca2+ concentration in HEK293 cells expressing each receptor, and that ligand selectivity of ghrelin with Ser-3 and Thr-3 that expected to see in the bullfrog receptor was not found in the two frog receptors, but in the newt receptor. The brain, gastrointestinal tract, kidney and gonad highly express GHS-R1a mRNA. In frogs and newt, fasting did not increase GHS-R1a mRNA expression in the brain, but in the stomach. However, intraperitoneal (IP) injection of ghrelin did not affect food intake. A dehydration treatment increased GHS-R1a mRNA expression in the brain, stomach and ventral skin in the tree frog. However, intracerebroventricular (ICV) injection of ghrelin did not affect water absorption. Ghrelin did not influence gastrointestinal motility in in vitro studies using smooth muscle strips of the bullfrog and newt in vitro.