Synchrotron Microtomography Applied to the Volumetric Analysis of Internal Structures of Thoropa Miliaris Tadpoles G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Karyological Study of Amphisbaena Ridleyi (Squamata, Amphisbaenidae), an Endemic Species of the Archipelago of Fernando De Noronha, Pernambuco, Brazil
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Genetics and Molecular Biology, 33, 1, 57-61 (2010) Copyright © 2009, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Short Communication Karyological study of Amphisbaena ridleyi (Squamata, Amphisbaenidae), an endemic species of the Archipelago of Fernando de Noronha, Pernambuco, Brazil Marcia Maria Laguna1, Renata Cecília Amaro2, Tamí Mott3, Yatiyo Yonenaga-Yassuda1 and Miguel Trefaut Rodrigues2 1Departamento de Genética e Biologia Evolutiva, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil. 2Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil. 3Instituto de Biociências, Programa de Pós Graduação em Ecologia e Conservação da Biodiversidade, Universidade Federal do Mato Grosso, Cuiabá, MT, Brazil. Abstract The karyotype of Amphisbaena ridleyi, an endemic species of the archipelago of Fernando de Noronha, in State of Pernambuco, Brazil, is described after conventional staining, Ag-NOR impregnation and fluorescence in situ hybrid- ization (FISH) with a telomeric probe. The diploid number is 46, with nine pairs of macrochromosomes (three metacentrics, four subtelocentrics and two acrocentrics) and 14 pairs of microchromosomes. The Ag-NOR is located in the telomeric region of the long arm of metacentric chromosome 2 and FISH revealed signals only in the telomeric region of all chromosomes. Further cytogenetic data on other amphisbaenians as well as a robust phylogenetic hy- pothesis of this clade is needed in order to understand the evolutionary changes on amphisbaenian karyotypes. Key words: Amphisbaena ridleyi, karyotype, Fernando de Noronha, Ag-NOR, FISH with telomeric probes. -

Anura: Brachycephalidae) Com Base Em Dados Morfológicos
Pós-graduação em Biologia Animal Laboratório de Anatomia Comparada de Vertebrados Departamento de Ciências Fisiológicas Instituto de Ciências Biológicas da Universidade de Brasília Sistemática filogenética do gênero Brachycephalus Fitzinger, 1826 (Anura: Brachycephalidae) com base em dados morfológicos Tese apresentada ao Programa de pós-graduação em Biologia Animal para a obtenção do título de doutor em Biologia Animal Leandro Ambrósio Campos Orientador: Antonio Sebben Co-orientador: Helio Ricardo da Silva Maio de 2011 Universidade de Brasília Instituto de Ciências Biológicas Programa de Pós-graduação em Biologia Animal TESE DE DOUTORADO LEANDRO AMBRÓSIO CAMPOS Título: “Sistemática filogenética do gêneroBrachycephalus Fitzinger, 1826 (Anura: Brachycephalidae) com base em dados morfológicos.” Comissão Examinadora: Prof. Dr. Antonio Sebben Presidente / Orientador UnB Prof. Dr. José Peres Pombal Jr. Prof. Dr. Lílian Gimenes Giugliano Membro Titular Externo não Vinculado ao Programa Membro Titular Interno Vinculado ao Programa Museu Nacional - UFRJ UnB Prof. Dr. Cristiano de Campos Nogueira Prof. Dr. Rosana Tidon Membro Titular Interno Vinculado ao Programa Membro Titular Interno Vinculado ao Programa UnB UnB Brasília, 30 de maio de 2011 Dedico esse trabalho à minha mãe Corina e aos meus irmãos Flávio, Luciano e Eliane i Agradecimentos Ao Prof. Dr. Antônio Sebben, pela orientação, dedicação, paciência e companheirismo ao longo do trabalho. Ao Prof. Dr. Helio Ricardo da Silva pela orientação, companheirismo e pelo auxílio imprescindível nas expedições de campo. Aos professores Carlos Alberto Schwartz, Elizabeth Ferroni Schwartz, Mácia Renata Mortari e Osmindo Pires Jr. pelos auxílios prestados ao longo do trabalho. Aos técnicos Pedro Ivo Mollina Pelicano, Washington José de Oliveira e Valter Cézar Fernandes Silveira pelo companheirismo e auxílio ao longo do trabalho. -

Congolius, a New Genus of African Reed Frog Endemic to The
www.nature.com/scientificreports OPEN Congolius, a new genus of African reed frog endemic to the central Congo: A potential case of convergent evolution Tadeáš Nečas1,2*, Gabriel Badjedjea3, Michal Vopálenský4 & Václav Gvoždík1,5* The reed frog genus Hyperolius (Afrobatrachia, Hyperoliidae) is a speciose genus containing over 140 species of mostly small to medium-sized frogs distributed in sub-Saharan Africa. Its high level of colour polymorphism, together with in anurans relatively rare sexual dichromatism, make systematic studies more difcult. As a result, the knowledge of the diversity and taxonomy of this genus is still limited. Hyperolius robustus known only from a handful of localities in rain forests of the central Congo Basin is one of the least known species. Here, we have used molecular methods for the frst time to study the phylogenetic position of this taxon, accompanied by an analysis of phenotype based on external (morphometric) and internal (osteological) morphological characters. Our phylogenetic results undoubtedly placed H. robustus out of Hyperolius into a common clade with sympatric Cryptothylax and West African Morerella. To prevent the uncovered paraphyly, we place H. robustus into a new genus, Congolius. The review of all available data suggests that the new genus is endemic to the central Congolian lowland rain forests. The analysis of phenotype underlined morphological similarity of the new genus to some Hyperolius species. This uniformity of body shape (including cranial shape) indicates that the two genera have either retained ancestral morphology or evolved through convergent evolution under similar ecological pressures in the African rain forests. African reed frogs, Hyperoliidae Laurent, 1943, are presently encompassing almost 230 species in 17 genera. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -
Amphibians of Santa Teresa, Brazil: the Hotspot Further Evaluated
A peer-reviewed open-access journal ZooKeys 857: 139–162 (2019)Amphibians of Santa Teresa, Brazil: the hotspot further evaluated 139 doi: 10.3897/zookeys.857.30302 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Amphibians of Santa Teresa, Brazil: the hotspot further evaluated Rodrigo Barbosa Ferreira1,2, Alexander Tamanini Mônico1,3, Emanuel Teixeira da Silva4,5, Fernanda Cristina Ferreira Lirio1, Cássio Zocca1,3, Marcio Marques Mageski1, João Filipe Riva Tonini6,7, Karen H. Beard2, Charles Duca1, Thiago Silva-Soares3 1Programa de Pós-Graduação em Ecologia de Ecossistemas, Universidade Vila Velha, Campus Boa Vista, 29102-920, Vila Velha, ES, Brazil 2 Department of Wildland Resources and the Ecology Center, Utah State University, Logan, UT, USA 3Instituto Nacional da Mata Atlântica/Museu de Biologia Prof. Mello Leitão, 29650-000, Santa Teresa, ES, Brazil 4 Laboratório de Herpetologia, Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Avenida Antônio Carlos, 6627, Pampulha, Belo Horizonte, MG, Brazil 5 Centro de Estudos em Biologia, Centro Universitário de Caratinga, Avenida Niterói, s/n, Bairro Nossa Senhora das Graças, 35300-000, Caratinga, MG, Brazil 6 Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA, USA 7 Museum of Comparative Zoology, Harvard University, 26 Oxford St, Cambridge, MA, USA Corresponding author: Rodrigo Barbosa Ferreira ([email protected]) Academic editor: A. Crottini | Received 4 October 2018 | Accepted 20 April 2019 | Published 25 June 2019 http://zoobank.org/1923497F-457B-43BA-A852-5B58BEB42CC1 Citation: Ferreira RB, Mônico AT, da Silva ET, Lirio FCF, Zocca C, Mageski MM, Tonini JFR, Beard KH, Duca C, Silva-Soares T (2019) Amphibians of Santa Teresa, Brazil: the hotspot further evaluated. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Seasonal and Habitat Structure of an Anuran Assemblage in A
An Acad Bras Cienc (2020) 92(1): e20190458 DOI 10.1590/0001-3765202020190458 Anais da Academia Brasileira de Ciências | Annals of the Brazilian Academy of Sciences Printed ISSN 0001-3765 I Online ISSN 1678-2690 www.scielo.br/aabc | www.fb.com/aabcjournal BIOLOGICAL SCIENCES Seasonal and habitat structure of an Running title: SEASONAL AND anuran assemblage in a semideciduous HABITAT STRUCTURE OF AN ANURAN ASSEMBLAGE forest area in Southeast Brazil Academy Section: BIOLOGICAL SCIENCES ELVIS A. PEREIRA, MATHEUS O. NEVES, JOSÉ LUIZ M.M. SUGAI, RENATO N. FEIO & DIEGO J. SANTANA e20190458 Abstract: In this study, we evaluated the reproductive activity and the temporal and spatial distributions of anuran assemblages in three environments within a semideciduous forest in Southeast Brazil, located at Municipality of Barão de Monte Alto, 92 State of Minas Gerais, Brazil. The fi eld activities were carried out during three consecutive (1) days, monthly throughout the rainy seasons of 2013–2014 and 2014–2015. We recorded 92(1) 28 anurans species, distributed in eight families. We observed the spatial-temporal distribution of some species, and their associated reproductive behaviors through exploration of vocalizations at different sites. The spatial and temporal distribution of the species seems to adapt to abiotic and biotic factors of their environment. Key words: Anuran community, community ecology, environmental heterogeneity, niche breadth, vocalization sites. INTRODUCTION community is defi ned as a group of organisms that coexist in a determined habitat and also Information about anuran habitat use and interact with one another and the surrounding reproductive ecology allows us to interpret environment (Begon et al. -
Figs1 Speciestree Seqcap
Phrynomantis microps Microhylidae Breviceps adspersus Brevicipitidae Hemisus marmoratus Hemisotidae Leptopelis rufus Cardioglossa gracilis Arthtroleptis poecilinotus Leptodactylodon ovatus Arthroleptidae Nyctibates corrugatus Trichobatrachus robustus Scotobleps gabonicus Acanthixalus spinosus Acanthixalus sonjae Semnodactylus wealli Paracassina obscura Paracassina kounhiensis Kassininae Phlyctimantis maculata Phlyctimantis verrucosus Phlyctimantis boulengeri Phlyctimantis leonardi Kassina kuvangensis Kassina fusca Kassina lamottei Kassina senegalensis Kassina decorata Kassina maculosa Kassina arboricola Hyperoliidae Kassina cochranae Opisthothylax immaculatus Afrixalus enseticola Tachycnemis seychellensis Heterixalus luteostriatus Malagasy-Seychelles Heterixalus alboguttatus Species Afrixalus vibekensis Afrixalus weidholzi Afrixalus lacustris Afrixalus dorsimaculatus 1 Afrixalus dorsimaculatus 2 Afrixalus knysae Afrixalus spinifrons Hyperoliinae Afrixalus delicatus Afrixalus sylvaticus Afrixalus brachycnemis Afrixalus laevis Afrixalus lacteus Afrixalus fornasini Afrixalus wittei Afrixalus osorioi Afrixalus paradorsalis paradorsalis 2 Afrixalus paradorsalis paradorsalis 1 Afrixalus paradorsalis manengubensis Afrixalus nigeriensis Afrixalus vittiger 1 Afrixalus vittiger 2 Afrixalus dorsalis 3 Afrixalus dorsalis 1 Afrixalus quadrivittatus 1 Afrixalus quadrivittatus 2 Afrixalus dorsalis 2 Afrixalus fulvovittatus 2 Afrixalus fulvovittatus 1 Morerella cyanophthalma Cryptothylax greshoffii Hyperolius semidiscus Hyperolius parkeri Hyperolius -

Early German Herpetological Observations and Explorations in Southern Africa, with Special Reference to the Zoological Museum of Berlin
Bonner zoologische Beiträge Band 52 (2003) Heft 3/4 Seiten 193–214 Bonn, November 2004 Early German Herpetological Observations and Explorations in Southern Africa, With Special Reference to the Zoological Museum of Berlin Aaron M. BAUER Department of Biology, Villanova University, Villanova, Pennsylvania, USA Abstract. The earliest herpetological records made by Germans in southern Africa were casual observations of common species around Cape Town made by employees of the Dutch East India Company (VOC) during the mid- to late Seven- teenth Century. Most of these records were merely brief descriptions or lists of common names, but detailed illustrations of many reptiles were executed by two German illustrators in the employ of the VOC, Heinrich CLAUDIUS and Johannes SCHUMACHER. CLAUDIUS, who accompanied Simon VAN DER STEL to Namaqualand in 1685, left an especially impor- tant body of herpetological illustrations which are here listed and identified to species. One of the last Germans to work for the Dutch in South Africa was Martin Hinrich Carl LICHTENSTEIN who served as a physician and tutor to the last Dutch governor of the Cape from 1802 to 1806. Although he did not collect any herpetological specimens himself, LICHTENSTEIN, who became the director of the Zoological Museum in Berlin in 1813, influenced many subsequent workers to undertake employment and/or expeditions in southern Africa. Among the early collectors were Karl BERGIUS and Ludwig KREBS. Both collected material that is still extant in the Berlin collection today, including a small number of reptile types. Because of LICHTENSTEIN’S emphasis on specimens as items for sale to other museums rather than as subjects for study, many species first collected by KREBS were only described much later on the basis of material ob- tained by other, mostly British, collectors. -

Rsidade Federal Do Paraná
UNIVERSIDADE FEDERAL DO PARANÁ CARLOS DANIEL RIVADENEIRA MONTENEGRO ECOLOGICAL IMPLICATIONS IN THE SPECIATION OF MONTANE FROGS WITH SKY ISLANDS DISTRIBUTION CURITIBA 2020 CARLOS DANIEL RIVADENEIRA MONTENEGRO ECOLOGICAL IMPLICATIONS IN THE SPECIATION OF MONTANE FROGS WITH SKY ISLANDS DISTRIBUTION Dissertação apresentada ao Programa de Pós-Graduação em Zoologia do Departamento de Zoologia, Setor de Ciências Biológicas da Universidade Federal do Paraná como requisito parcial para a obtenção do título de Mestre em Zoologia. Orientador: Prof. Dr. Marcio R. Pie Co-orientador: Dr. Andreas L. S. Meyer CURITIBA 2020 Universidade Federal do Paraná. Sistema de Bibliotecas. Biblioteca de Ciências Biológicas. (Rosilei Vilas Boas – CRB/9-939). Montenegro, Carlos Daniel Rivadeneira. Ecological implications in the speciation of montane frogs with sky islands distribution. / Carlos Daniel Rivadeneira Montenegro. – Curitiba, 2020. 51 f. : il. Orientador: Marcio R. Pie. Coorientador: Andreas L. S. Meyer. Dissertação (Mestrado) – Universidade Federal do Paraná, Setor de Ciências Biológicas. Programa de Pós-Graduação em Zoologia. 1. Sapo. 2. Herpetologia. 3. Habitat (Ecologia). 4. Nicho (Ecologia). 5. Anfíbio – Mata Atlântica. I.Título. II. Pie, Marcio R. III. Meyer, Andreas L. S. IV. Universidade Federal do Paraná. Setor de Ciências Biológicas. Programa de Pós-Graduação em Zoologia. CDD (20.ed.) 597.8 MINISTÉRIO DA EDUCAÇÃO SETOR DE CIENCIAS BIOLOGICAS UNIVERSIDADE FEDERAL DO PARANÁ PRÓ-REITORIA DE PESQUISA E PÓS-GRADUAÇÃO PROGRAMA DE PÓS-GRADUAÇÃO ZOOLOGIA - 40001016008P4 TERMO DE APROVAÇÃO Os membros da Banca Examinadora designada pelo Colegiado do Programa de Pós-Graduação em ZOOLOGIA da Universidade Federal do Paraná foram convocados para realizar a arguição da Dissertação de Mestrado de CARLOS DANIEL RIVADENEIRA MONTENEGRO intitulada: Ecological implications in the speciation of montane frogs with sky islands distribution, sob orientação do Prof. -
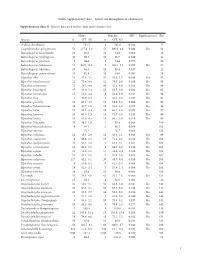
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Check List and Authors
ISSN 1809-127X (online edition) www.checklist.org.br Journal of Species Lists and Distribution © 2009 Check List and Authors LISTS OF SPECIES Anurans in bromeliads, Parque Estadual da Serra do Brigadeiro, state of Minas Gerais, southeastern Brazil João Victor Andrade de Lacerda,* Breno de Assis, Diego José Santana, and Renato Neves Feio Universidade Federal de Viçosa, Departamento de Biologia Animal, Museu de Zoologia João Moojen. CEP 36570-000. Viçosa, MG, Brazil. * Corresponding author. E-mail: [email protected] Abstract: This study presents a list of anurans associated to bromeliads in Parque Estadual da Serra do Brigadeiro, state of Minas Gerais, southeastern Brazil. It also brings information regarding the type of association between these anurans and plants. We recorded eight species belonging to five genera and two families, Cycloramphidae and Hylidae. The most abundant species was Scinax gr. perpusillus, followed by Scinax aff. perereca, Dendropsophus minutus, Bokermannohyla circumdata, Hypsiboas faber, Thoropa miliaris, Hypsiboas polytaenius and Dendropsophus elegans. Most species observed occupying bromeliads uses these microhabitats only as diurnal shelter. Scinax gr. perpusillus was the only classified as bromeligenous, depending upon the plants to complete its reproductive cycle. Introduction shelter but its reproductive cycle is not dependent Most species of amphibians have nocturnal habits on the plant while the bromeligenous are strictly and reproduce in the warmer and wet season of dependent on the plant, where they spend the the year, avoiding exposure to diurnal higher entire life cycle. temperatures and lower atmospheric humidity (Duellman and Trueb 1994; Pombal Jr. 1997). This study presents a list of anurans associated to Anurans, in the course of their evolution, bromeliads in the central region of Parque colonized much diversified types of microhabitats Estadual da Serra do Brigadeiro, in (Pertel et al.