Auditory Input Enhances Somatosensory Encoding and Tactile Goal-Directed Behavior ✉ L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -
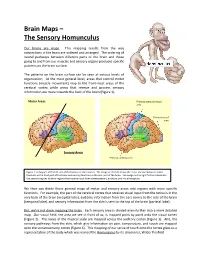
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -
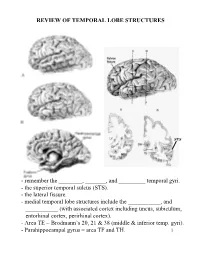
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

2018 Neuromodulation of Right Auditory Cortex
Neuromodulation of right auditory cortex selectively increases activation ANGOR UNIVERSITY in speech-related brain areas in brainstem auditory agnosia Bestelmeyer, Patricia E.G.; Davis, Nick J.; Poliva, Oren; Rafal, Robert D. Cognitive and Behavioral Neurology DOI: 10.1097/WNN.0000000000000162 PRIFYSGOL BANGOR / B Published: 01/09/2018 Peer reviewed version Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Bestelmeyer, P. E. G., Davis, N. J., Poliva, O., & Rafal, R. D. (2018). Neuromodulation of right auditory cortex selectively increases activation in speech-related brain areas in brainstem auditory agnosia. Cognitive and Behavioral Neurology, 31(3), 151-155. https://doi.org/10.1097/WNN.0000000000000162 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 27. Sep. 2021 Anodal tDCS over auditory cortex modulates neural activation in auditory agnosia Neuromodulation of right auditory cortex selectively increases activation in speech-related brain areas in brainstem auditory agnosia Patricia E.G. -

Functional Topography of Human Auditory Cortex
1416 • The Journal of Neuroscience, January 27, 2016 • 36(4):1416–1428 Behavioral/Cognitive Functional Topography of Human Auditory Cortex Amber M. Leaver1,2 and Josef P. Rauschecker1,3 1Laboratory of Integrative Neuroscience and Cognition, Department of Neuroscience, Georgetown University Medical Center, Washington, DC 20007, 2Department of Neurology, Ahmanson-Lovelace Brain Mapping Center, Los Angeles, California 90095, and 3Institute for Advanced Study, Technische Universita¨t Mu¨nchen, D-85748 Garching, Germany Functional and anatomical studies have clearly demonstrated that auditory cortex is populated by multiple subfields. However, func- tional characterization of those fields has been largely the domain of animal electrophysiology, limiting the extent to which human and animal research can inform each other. In this study, we used high-resolution functional magnetic resonance imaging to characterize human auditory cortical subfields using a variety of low-level acoustic features in the spectral and temporal domains. Specifically, we show that topographic gradients of frequency preference, or tonotopy, extend along two axes in human auditory cortex, thus reconciling historical accounts of a tonotopic axis oriented medial to lateral along Heschl’s gyrus and more recent findings emphasizing tonotopic organization along the anterior–posterior axis. Contradictory findings regarding topographic organization according to temporal mod- ulation rate in acoustic stimuli, or “periodotopy,” are also addressed. Although isolated subregions show a preference for high rates of amplitude-modulated white noise (AMWN) in our data, large-scale “periodotopic” organization was not found. Organization by AM rate was correlated with dominant pitch percepts in AMWN in many regions. In short, our data expose early auditory cortex chiefly as a frequency analyzer, and spectral frequency, as imposed by the sensory receptor surface in the cochlea, seems to be the dominant feature governing large-scale topographic organization across human auditory cortex. -

Neuromodulation and Plasticity of the Adult Auditory Cortex
Cosyne 2007 Friday evening, Poster II-52 Neuromodulation and plasticity of the adult auditory cortex RC Froemke1, P Levis2, MM Merzenich1, CE Schreiner1 1: Coleman Lab, Keck Center, Dept. Otolaryngology, UCSF, CA, USA 2:Computer Systems Lab, Depts. EE & CS, Stanford University, Palo Alto, CA, USA Cortical networks are highly dynamic and labile. In large part, these qualities reflect the ability of cortical synapses to be rapidly modified in a way that depends on the patterns of experience, activity, and neuromodulation. However, the rules of cortical synaptic plasticity and neuromodulation remain unclear, especially in the intact brain, where cortical circuitry is under the powerful influence of a diverse set of subcortical systems. To understand how neuronal activity and neuromodulation lead to changes in cortical synapses in vivo, we have used a combination of approaches, including in vivo whole-cell recording, electrical stimulation of the cortex, thalamus, and subcortical neuromodulator nuclei, and telemetric recording and stimulation in the behaving animal. In the experiments reported here, we have focused on the organization of receptive fields in the primary auditory cortex (A1) of adult rats, and the control of cortical responses by modulatory inputs from the basal forebrain. Our whole-cell recording experiments first showed that sensory stimulation alone does not lead to long-term changes in synaptic receptive fields, suggesting that repetitive pre- and postsynaptic spiking (such as that which drives spike-timing-dependent plasticity) are not sufficient for long- term synaptic modification in adult A1 in vivo. However, pairing sensory stimulation with electrical stimulation of the basal forebrain, containing the major cholinergic input to the cortex, reliably produced large long-term changes in the synaptic receptive fields of A1 neurons.