Synopsis of Fossil Fish Fauna from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pacific Plate Biogeography, with Special Reference to Shorefishes
Pacific Plate Biogeography, with Special Reference to Shorefishes VICTOR G. SPRINGER m SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 367 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -
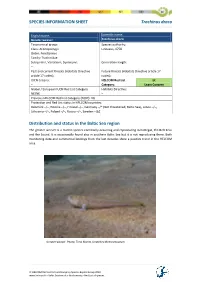
HELCOM Red List
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

A Framework of Ichthyofaunal Ecostratigraphy of the Oligocene–Early Miocene Strata of the Polish Outer Carpathian Basin
Annales Societatis Geologorum Poloniae (2006), vol. 76: 1–111. A FRAMEWORK OF ICHTHYOFAUNAL ECOSTRATIGRAPHY OF THE OLIGOCENE–EARLY MIOCENE STRATA OF THE POLISH OUTER CARPATHIAN BASIN Janusz KOTLARCZYK1, Anna JERZMAÑSKA2, Ewa ŒWIDNICKA2 & Teresa WISZNIOWSKA2 1 Department of General and Mathematical Geology, AGH University of Science and Technology, Al. Mickiewicza 30, 30-059 Kraków, Poland 2 Department of Palaeozoology, Institute of Zoology, University of Wroc³aw, Sienkiewicza, 21, 50-335 Wroc³aw, Poland Kotlarczyk, J., Jerzmañska, A., Œwidnicka, E. & Wiszniowska, T., 2006. A framework of ichthyofaunal ecostrati- graphy of the Oligocene–Early Miocene strata of the Polish Outer Carpathian basin. Annales Societatis Geologorum Poloniae, 76: 1–111. Abstract: The paper presents the results of an analysis of ichthyofaunal variability throughout the section of the Menilite-Krosno Series (MKS) in the Outer Carpathians of Poland. The studied tanathocoenoses were formed at the bottom of a more than 2,000 m deep northern basin of the Tethys, being largely represented by the continental rise and bottoms of its narrow furrows, and – to a lesser degree – the continental slope and slopes of a submarine high. Lateral variability of statististically representative assemblages of tanathocoenoses hosted in thin, isochro- nous horizons is interpreted as a result of both local changes of ichthyocoenoses and the influence of post-mortem relocation of fishes that mainly dwelled the shelf and upper continental slope. Vertical variability, in turn, is considered as a resulting from changeable conditions of the ecological environment, the input and outflow of taxa whose evolution proceeded in the Indo-Pacific area, and the species extinction. Changeability of ichthyofauna within a ca. -

Revisión Taxonómica De La Ictiología Marina De Galicia: Clase Actinopteri (Orden Trachiniformes Al Orden Tetraodontiformes)
Nova Acta Científica Compostelana (Bioloxía), 28: 77-104 (2021) - ISSN 2340-0021 ARTÍCULO DE INVESTIGACIÓN Revisión taxonómica de la ictiología marina de Galicia: Clase Actinopteri (Orden Trachiniformes al Orden Tetraodontiformes) Taxonomic review of Galician marine ichthyology: Classe Actinopteri (Order Trachiniformes to Order Tetraodontiformes) *RAFAEL BAÑÓN, TOÑO MAÑO Grupo de Estudos do Medio Mariño (GEMM), Puerto Deportivo s/n 15960 Ribeira, A Coruña, España. *[email protected]; [email protected] (Recibido 29/11/2020; Aceptado 26/03/2021) Resumen En este trabajo se realiza una revisión taxonómica de los peces óseos de Galicia (Clase Actinopteri) del Orden Trachiniformes al Orden Tetraodontiformes, a través de los distintos tratados y publicaciones ictio- lógicas publicadas a lo largo de la historia. Se listan un total de 188 especies, de las cuales 5 se consideran dudosas, al no estar su presencia suficientemente demostrada. Una revisión de la bibliografía y nomenclatura científica nos ha permitido citar nuevas especies para Galicia y reasignar antiguas denominaciones a nuevas especies, subsanando errores de identificación de otros autores. El orden Perciformes, con 145 especies, es el más numeroso de los peces de Galicia. A este orden pertenecen especies de alto interés comercial como el jurel Trachurus trachurus y la caballa Scomber scombrus. El listado incluye también los primeros registros para Galicia de especies de carácter tropical desplazadas hacia el norte debido al cambio climático a lo largo de estas últimas décadas. Algunas de estas especies son el jurelo azul Caranx crysos, el pez globo Lagocephalus laevigatus y el mero tropical Epinephelus aeneus. Palabras clave: Peces óseos, nomenclatura, ictiología, tropicalización. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Title the ZOOGEOGRAPHICAL ASPECTS of the JAPAN
THE ZOOGEOGRAPHICAL ASPECTS OF THE JAPAN Title SEA -PART V- Author(s) Nishimura, Saburo PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1969), 17(2): 67-142 Issue Date 1969-09-30 URL http://hdl.handle.net/2433/175589 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University THE ZOOGEOGRAPHICAL ASPECTS OF THE JAPAN SEA 1 PART V ) SABURO NISHIMURA Seta Marine Biological Laboratory, Sirahama With Text-figures 31-48 CONTENTS Page 4. Distribution of Animal Populations in Relation to the Hydrography of the Japan Sea ...................·............................................................................................... 67 4.1. The Oceanographic Structure and Animal Communities . ....................................... 68 4.2. Movement and Transformation of "Vater Masses and the Migrations of Animal Populations . 82 4.2.1. Winter to Spring Aspects . .. .. .. .. 82 4.2.2. Summer Aspects .... .... ............. ... .. ....... .... ....... ....... ................. ............. .. .... 92 4.2.3. Autumnal Aspects . .. .. .. .. 99 4.2.4. Returning to the Winter Aspects .................................................................. 101 5. Characteristics in Biological Production in the Japan Sea . I 05 5.1. Characteristics in Plankton Production ................................................................ 105 5.2. Biological Seasons and Bioclimatic Regions .......................................................... II 0 5.3. Fate of Immigrant Southern and Northern Populations ......................................... -

Otolith Atlas for the Western Mediterranean, North and Central Eastern Atlantic
SCIENTIA MARINA 72S1 July 2008, 7-198, Barcelona (Spain) ISSN: 0214-8358 Otolith atlas for the western Mediterranean, north and central eastern Atlantic VICTOR M. TUSET 1, ANTONI LOMBARTE 2 and CARLOS A. ASSIS 3 1 Instituto Canario de Ciencias Marinas, Departamento de Biología Pesquera, P.O. Box. 56, E-35200 Telde (Las Palmas), Canary Islands, Spain. E-mail: [email protected] 2 Institut de Ciències del Mar-CSIC, Departament de Recursos Marins Renovables, Passeig Marítim 37-49, Barcelona 08003, Catalonia, Spain. 3 Instituto de Oceanografia e Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, Campo Grande 1749-016, Lisboa, Portugal. SUMMARY: The sagittal otolith of 348 species, belonging to 99 families and 22 orders of marine Teleostean fishes from the north and central eastern Atlantic and western Mediterranean were described using morphological and morphometric characters. The morphological descriptions were based on the otolith shape, outline and sulcus acusticus features. The mor- phometric parameters determined were otolith length (OL, mm), height (OH, mm), perimeter (P; mm) and area (A; mm2) and were expressed in terms of shape indices as circularity (P2/A), rectangularity (A/(OL×OH)), aspect ratio (OH/OL; %) and OL/fish size. The present Atlas provides information that complements the characterization of some ichthyologic taxa. In addition, it constitutes an important instrument for species identification using sagittal otoliths collected in fossiliferous layers, in archaeological sites or in feeding remains of bony fish predators. Keywords: otolith, sagitta, morphology, morphometry, western Mediterranean, north eastern Atlantic, central eastern Atlantic. RESUMEN: Otolitos de peces del mediterráneo occidental y del atlántico central y nororiental. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -

BULLETIN FLORIDA STATE MUSEUM Vol
a BULLETIN OF THE FLORIDA STATE MUSEUM BIOLOGICAL SCIENCES Volume 3 Number 3 A REVISION OF THE FISHES OF THE SUBFAMILY ARGENTININAE ) ■. ) iz e? Daniel M. Cohen ssfiqo UNIVERSITY OF FLORIDA Gainesville 1958 The numbers of THE BULLETIN OF THE FLORIDA STATE MUSEUM, BIOLOGICAL SCIENCES, will be published at irregular intervals. Volumes will contain about 300 pages and will not necessarily be completed in any one calendar year. WILLIAM J. RIEMER, Editor ROLAND F. HUSSEY, Associate Editor All communications concerning purchase or exchange of the publication should be addressed to the Curator of Biological Sciences, Florida State Museum, Seagle Building, Gainesville, Florida. Manuscripts should be sent to the Editor of the BULLETIN, Flint Hall, University of Florida, Gainesville, Florida. Published 2 July 1958 Price for this issue $1.14 A REVISION OF THE FISHES OF THE SUBFAMILY ARGENTININAE 1 DANIEL M. COHEN SYNOPSIS: A systematic revision of the Argentininae divides the subfamily into two genera, Argentina with seven species and subspecies and Glossanodon with five species. Comments are given on habits and distribution, and brief dis- cussions of the swimbladder and the spiral valve are included. The following new taxa are presented: Prosoarchus, new subgenus; Glossanodon (Glossanodon) polli, new species; Glossanodon (Prosoarchus) pygmaeus, new species; Argentina elon- gata austrahae, new subspecies. INTRODUCTION The subfamily Argentininae as herein understood consists of argen- tinid fishes formerly referable to the genus Argentina. This subfamily is worldwide in distribution, and its members, as adults at least, are restricted to the edges and slopes of the continental shelf, in contra- distinction to the other fishes of the suborder Argentinoidei which are bathypelagic. -

Fish Otoliths from the Pre-Evaporitic Early Messinian of Northern Italy: Their Stratigraphic and Palaeobiogeographic Significance
Facies (2010) 56:399-432 DO1 10.1007/s10347-010-0212-6 Fish otoliths from the pre-evaporitic Early Messinian of northern Italy: their stratigraphic and palaeobiogeographic significance Angela Girone a Dirk Nolf * Oreste Cavallo Received: 13 August 2009 / Accepted: 4 January 2010 / Published online: 9 February 2010 O Springer-Verlag 2010 Abstract The study of otolith assemblages from the pre- affinity of the fossil assemblage with the present-day Medi- evaporitic Messinian deposits allows the reconstruction of a terranean neritic fauna, which was already recorded at the fauna of 79 taxa of which 35 could be identified at the spe- genus level for the Rupelian fauna, persists during the Neo- cific level. Three of these are new: Diaphus rubus, Myctop- gene and continues until the Pleistocene. hum coppa, and Uranoscopus ciabatta. The assemblages reflect mainly a neritic environment influenced by the oce- Kepords Fishes . Teleostei . Otoliths . Messinian anic realm. Analysis of the global present-day geographic Appearance . Extinction distribution of 42 of the recognised Messinian genera indi- cates that 88% of these are still living in the Mediterranean, 98% in the Atlantic and 78% in the Indo-Pacific realm. Introduction These results are in good agreement with the evolutionary trends documented for the Oligocene and Miocene teleost During the Late Miocene (Tortonian and Messinian), the fauna, specifically an increase in percentage of genera Tethyan Ocean was ultimately closed as result of synoro- inhabiting the modern Mediterranean, a very high percent- genic collisional tectonism, and its Mesozoic and Cenozoic age of Atlantic and Indo-Pacific genera, and a slight fall of sedimentary sequences were deformed and uplifted along the importance of present-day Indo-Pacific genera from the the emerging Alpine-Himalayan orogenic system. -

Andrew David Dorka Cobián Rojas Felicia Drummond Alain García Rodríguez
CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington ACKNOWLEDGMENTS This research was supported by the NOAA Office of Ocean Exploration and Research under award number NA14OAR4320260 to the Cooperative Institute for Ocean Exploration, Research and Technology (CIOERT) at Harbor Branch Oceanographic Institute-Florida Atlantic University (HBOI-FAU), and by the NOAA Pacific Marine Environmental Laboratory under award number NA150AR4320064 to the Cooperative Institute for Marine and Atmospheric Studies (CIMAS) at the University of Miami. This expedition was conducted in support of the Joint Statement between the United States of America and the Republic of Cuba on Cooperation on Environmental Protection (November 24, 2015) and the Memorandum of Understanding between the United States National Oceanic and Atmospheric Administration, the U.S. National Park Service, and Cuba’s National Center for Protected Areas. We give special thanks to Carlos Díaz Maza (Director of the National Center of Protected Areas) and Ulises Fernández Gomez (International Relations Officer, Ministry of Science, Technology and Environment; CITMA) for assistance in securing the necessary permits to conduct the expedition and for their tremendous hospitality and logistical support in Cuba. We thank the Captain and crew of the University of Miami R/V F.G. Walton Smith and ROV operators Lance Horn and Jason White, University of North Carolina at Wilmington (UNCW-CIOERT), Undersea Vehicle Program for their excellent work at sea during the expedition. -

Nika STAGLIČIĆ, Branko DRAGIČEVIĆ*, Iva ŽUŽUL, and Tanja ŠEGVIĆ-BUBIĆ
ACTA ICHTHYOLOGICA ET PISCATORIA (2019) 49 (2): 177–180 DOI: 10.3750/AIEP/02464 ANOMALOUS COLOURATION OF A STARRY WEEVER, TRACHINUS RADIATUS (ACTINOPTERYGII: PERCIFORMES: TRACHINIDAE), FROM THE ADRIATIC SEA Nika STAGLIČIĆ, Branko DRAGIČEVIĆ*, Iva ŽUŽUL, and Tanja ŠEGVIĆ-BUBIĆ Institute of Oceanography and Fisheries, Split, Croatia Stagličić N., Dragičević B., Žužul I., Šegvić-Bubić T. 2019. Anomalous colouration of a starry weever, Trachinus radiatus (Actinopterygii: Perciformes: Trachinidae), from the Adriatic Sea. Acta Ichthyol. Piscat. 49 (2): 177–180. Abstract. The record of abnormally pigmented starry weever, Trachinus radiatus Cuvier, 1829, is described. The specimen was caught in Nature Park Lastovo archipelago, eastern Adriatic Sea, and displayed an overall lack of usual dark brown pigmentation. The skin was marked by a predominance of yellow to red pigments corresponding with a rare colour anomaly condition of xanthochromism. Due to the unusual appearance of the specimen, we used molecular methods for proper identification of the species. This seems to be the first documented record of xanthochromism in the family Trachinidae. Keywords: xanthochromism, abnormal pigmentation, abnormal colouration, Adriatic Sea, Trachinus, Nature Park Lastovo INTRODUCTION of melanophores or other pigment cells. Aberrant colour Fish display a remarkable variety of body colours and pigment patterns are particularly common in aquacul- and patterns that differ both within and between species ture, where they create a problem of reduced marketability (Fujii 1993, Mills and Patterson 2009). Colouration and (Leclercq et al. 2010, Wishkerman et al. 2016). Compared patterning are traits with high ecological significance to rearing conditions, cases of abnormal pigmentation and serve diverse functions in that sense, e.g., predation are much rarer in wild populations (Venizelos and Ben- avoidance, mate recognition and choice, camouflage, etti 1999, Wishkerman et al.