Download (2MB)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preserving the Tree of Life of the Fish Family Cyprinidae in Africa in the Face of the Ongoing Extinction Crisis
Genome Preserving the tree of life of the fish family Cyprinidae in Africa in the face of the ongoing extinction crisis Journal: Genome Manuscript ID gen-2018-0023.R3 Manuscript Type: Article Date Submitted by the 02-Mar-2019 Author: Complete List of Authors: Adeoba, Mariam; University of Johannesburg Tesfamichael, Solomon; University of Johannesburg Yessoufou, Kowiyou; University of Johannesburg Conservation, African freshwater Ecosystems, IUCN Red List, EDGE, DNA Keyword: Draft barcoding Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 42 Genome Preserving the tree of life of the fish family Cyprinidae in Africa in the face of the ongoing extinction crisis Mariam Salami1, Solomon Tesfamichael2, Kowiyou Yessoufou2 1Department of Zoology, University of Johannesburg, Kingsway Campus, PO Box 524, Auckland Park 2006, South Africa 2Department of Geography, Environmental Management and Energy studies, University of Johannesburg, Kingsway Campus, PO Box 524, Auckland Park 2006, South Africa *Corresponding author: Kowiyou Yessoufou [email protected] Draft 1 https://mc06.manuscriptcentral.com/genome-pubs Genome Page 2 of 42 Abstract Our understanding of how the phylogenetic tree of fishes might be affected by the ongoing extinction risk is poor. This is due to the unavailability of comprehensive DNA data, especially for many African lineages. In addition, the ongoing taxonomic confusion within some lineages, e.g. Cyprinidae, makes it difficult to contribute to the debate on how the fish tree of life might be shaped by extinction. Here, we combine COI sequences and taxonomic information to assemble a fully sampled phylogeny of the African Cyprinidae and investigate whether we might lose more phylogenetic diversity (PD) than expected if currently-threatened species go extinct. -

Reproductive Behavior, Development and Eye Regression in the Cave
Neotropical Ichthyology, 7(3):479-490, 2009 Copyright © 2009 Sociedade Brasileira de Ictiologia Reproductive behavior, development and eye regression in the cave armored catfish, Ancistrus cryptophthalmus Reis, 1987 (Siluriformes: Loricariidae), breed in laboratory Sandro Secutti and Eleonora Trajano The troglobitic armored catfish, Ancistrus cryptophthalmus (Loricariidae, Ancistrinae) is known from four caves in the São Domingos karst area, upper rio Tocantins basin, Central Brazil. These populations differ in general body shape and degree of reduction of eyes and of pigmentation. The small Passa Três population (around 1,000 individuals) presents the most reduced eyes, which are not externally visible in adults. A small group of Passa Três catfish, one male and three females, reproduced spontaneously thrice in laboratory, at the end of summertime in 2000, 2003 and 2004. Herein we describe the reproductive behavior during the 2003 event, as well as the early development of the 2003 and 2004 offsprings, with focus on body growth and ontogenetic regression of eyes. The parental care by the male, which includes defense of the rock shelter where the egg clutch is laid, cleaning and oxygenation of eggs, is typical of many loricariids. On the other hand, the slow development, including delayed eye degeneration, low body growth rates and high estimated longevity (15 years or more) are characteristic of precocial, or K-selected, life cycles. In the absence of comparable data for close epigean relatives (Ancistrus spp.), it is not possible to establish whether these features are an autapomorphic specialization of the troglobitic A. cryptophthalmus or a plesiomorphic trait already present in the epigean ancestor, possibly favoring the adoption of the life in the food-poor cave environment. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Clarias Gariepinus (Burchell, 1822)
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department Cultured Aquatic Species Information Programme Clarias gariepinus (Burchell, 1822) I. Identity V. Status And Trends a. Biological Features VI. Main Issues b. Images Gallery a. Responsible Aquaculture Practices II. Profile VII. References a. Historical Background a. Related Links b. Main Producer Countries c. Habitat And Biology III. Production a. Production Cycle b. Production Systems c. Diseases And Control Measures IV. Statistics a. Production Statistics b. Market And Trade Identity Clarias gariepinus Burchell, 1822 [Clariidae] FAO Names: En - North African catfish, Fr - Poisson-chat nord-africain, Es - Pez-gato Biological features Body elongate. Head large, depressed and bony with small eyes. Narrow and angular occipital process; gill openings wide; air-breathing labyrinthic organ arising from gill arches; first gill arch with 24 to 110 gillrakers; cleithrum pointed, narrow with longitudinal ridges and with sharpness. Mouth terminal, large. Four pairs of barbels present. Long dorsal and anal fins; without dorsal fin spine and adipose fin. Anterior edge of pectoral spine serrated. Caudal fin rounded. Colour varies from sandy-yellow through gray to olive with dark greenish-brown markings, belly white. View FAO FishFinder Species fact sheet Images gallery FAO Fisheries and Aquaculture Department 2.5 kg Clarias gariepinus Nursing semi-intensive pond Clarias fry nursing tank Clarias intensive nursing (Photo: John Moehl) Clarias intensive farming Clarias harvest in Cameroon Profile Historical background African catfish are mentioned within traditional capture-based aquaculture (known as wheddos in Benin and Ghana and barochois in Mauritius) for centuries. Their culture in modern times follows a similar trend to that of tilapias: first domestication trials by the year 1950 and adoption of the North African catfish Clarias gariepinus as the most desirable catfish for aquaculture in the mid 1970s. -

A Guide to the Parasites of African Freshwater Fishes
A Guide to the Parasites of African Freshwater Fishes Edited by T. Scholz, M.P.M. Vanhove, N. Smit, Z. Jayasundera & M. Gelnar Volume 18 (2018) Chapter 2.1. FISH DIVERSITY AND ECOLOGY Martin REICHARD Diversity of fshes in Africa Fishes are the most taxonomically diverse group of vertebrates and Africa shares a large portion of this diversity. This is due to its rich geological history – being a part of Gondwana, it shares taxa with the Neotropical region, whereas recent close geographical affnity to Eurasia permitted faunal exchange with European and Asian taxa. At the same time, relative isolation and the complex climatic and geological history of Africa enabled major diversifcation within the continent. The taxonomic diversity of African freshwater fshes is associated with functional and ecological diversity. While freshwater habitats form a tiny fraction of the total surface of aquatic habitats compared with the marine environment, most teleost fsh diversity occurs in fresh waters. There are over 3,200 freshwater fsh species in Africa and it is likely several hundreds of species remain undescribed (Snoeks et al. 2011). This high diversity and endemism is likely mirrored in diversity and endemism of their parasites. African fsh diversity includes an ancient group of air-breathing lungfshes (Protopterus spp.). Other taxa are capable of breathing air and tolerate poor water quality, including several clariid catfshes (e.g., Clarias spp.; Fig. 2.1.1D) and anabantids (Ctenopoma spp.). Africa is also home to several bichir species (Polypterus spp.; Fig. 2.1.1A), an ancient fsh group endemic to Africa, and bonytongue Heterotis niloticus (Cuvier, 1829) (Osteoglossidae), a basal actinopterygian fsh. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

African Sharptooth Catfish Clarias Gariepinus
African sharptooth catfish Clarias gariepinus 1 Taxonomy Species: Clarias gariepinus (Burchell, 1822) Family: Clariidae Order: Siluriformes Class: Actinopterygii African sharptooth catfish Clarias gariepinus is a typical air-breathing catfish with a scaleless, bony elongated body with long dorsal and anal fins, and a helmet like head (Figure 1). Colour varies dorsally from dark to light brown and is often mottled with shades of olive and grey while the underside is a pale cream to white (Skelton 2001). It can grow very large with a maximum reported length of 170 cm (IGFA 2001) and weight of 60 kg (Robbins et al. 1991). Figure 1. Lateral view of Clarias gariepinus (Source: FAO 2012). The genus Clarias was reviewed in the 1980s, which resulted in several widespread species being synonymized (Clarias capensis of southern Africa, C. mossambicus of central Africa and C. lazera of west and north Africa) under the name Clarias gariepinus (Teugels 1986). 2 Natural distribution and habitat The native range of C. gariepinus covers most of the African continent, with the exception of Maghreb, Upper and Lower Guinea, and the Cape provinces of South Africa (Picker & Griffiths 2011) (Figure 2). According to Skelton (2001) it is probably the most widely distributed fish in Africa. Jubb (1967) describes its natural distribution as occurring as far south as the Orange River system in the west and the Umtamvuna River in the east of South Africa. Page | 1 C. gariepinus is widely tolerant of many different habitats, even the upper reaches of estuaries, but is considered to be a freshwater species. It favours floodplains, slow flowing rivers, lakes and dams (Skelton 2001). -

Clarias Batrachus (Siluriformes, Clariidae) in Madura Island, Indonesia 1Ihwan, 2Fajar S
Presence of Asian catfish Clarias batrachus (Siluriformes, Clariidae) in Madura Island, Indonesia 1Ihwan, 2Fajar S. Pratama, 3Danang Yonarta, 4Abdul R. Faqih, 4Maheno S. Widodo, 5Fitri S. Valen, 5Muhammad B. Tamam, 5,6Veryl Hasan 1 Marine and Fisheries Polytechnic of Bone, Aquaculture Technology Study Program, Bone, South Sulawesi, Indonesia; 2 Ministry of Marine Affairs and Fisheries Republic of Indonesia, Directorate General of Marine and Fisheries Resources Surveillance, Tual Surveillance for Marine and Fisheries Resources Base, Tual, Maluku, Indonesia; 3 Study Programe of Aquaculture, Faculty of Agriculture, University of Sriwijaya, Palembang- Prabumulih street, Ogan Ilir, South Sumatra, Indonesia; 4 Brawijaya University, Fisheries and Marine Science Faculty, Aquatic Resources Management Department, Veteran Malang, East Java, Indonesia; 5 Biology Generation Indonesia Foundation, Zoology Division, Gresik, East Java, Indonesia; 6 Airlangga University, Fisheries and Marine Faculty, Fish Health Management and Aquaculture Department, Surabaya, East Java, Indonesia. Corresponding author: V. Hasan, [email protected] Abstract. Clarias batrachus (Linnaeus, 1758) was a species that its condition faced the risk of extinction in most parts the Java mainland due to the African catfish Clarias gariepinus (Burchell, 1822) culture industry. In September 2018, C. batrachus was captured and photographed in the Saroka River, Madura Island, east and of Java mainland. The morphological characters of this species confirm its presence in a new river, more than 150 km northeast from its type locality. The specimens of C. batrachus were characterized as follows: dorsal fin rays 60-66; anal fin rays 47-50; pectoral fin rays 9-11; ventral fin rays 6. Key Words: walking catfish, distribution, native fish, freshwater fish. -

A GIS Assessment of the Suitability of Tilapia and Clarias Pond Farming in Tanzania
International Journal of Geo-Information Article A GIS Assessment of the Suitability of Tilapia and Clarias Pond Farming in Tanzania Håkan Berg 1,* , Deogratias Mulokozi 2 and Lars Udikas 1 1 Department of Physical Geography, Stockholm University, 106 91 Stockholm, Sweden; [email protected] 2 Kigoma Centre, Tanzania Fisheries Research Institute, Kigoma P.O. Box 90, Tanzania; deogratiasmulokozi@tafiri.go.tz * Correspondence: [email protected] Abstract: Aquaculture production in Tanzania has increased in recent years, responding to an increased demand for fish, but the scale and productivity of smallholder aquaculture remains below the level needed to support significant sector growth in Tanzania. This study assesses, through geospatial analyses, the suitability for freshwater pond farming of Oreochromis niloticus and Clarias gariepinus in Tanzania, by assessing the geographical distribution of seven criteria (water availability, water temperature, soil texture, terrain slope, availability of farm inputs, potential farm-gate sales, and access to local markets) identified as important for fish pond farming. The criteria were developed and standardized from 15 sub-criteria, which were classified into a four-level suitability scale based on physical scores. The individual weights of the different criteria in the overall GIS suitability assessment were determined through a multi-criteria evaluation. The final results were validated and compared through field observations, interviews with 89 rural and 11 urban aquaculture farmers, and a questionnaire survey with 16 regional fisheries officers. Our results indicate that there is a good potential for aquaculture in Tanzania. Almost 60% of Tanzania is assessed as being suitable and Citation: Berg, H.; Mulokozi, D.; 40% as moderately suitable for small-scale subsistence pond farming, which is the dominating fish Udikas, L. -

Etude Comparã©E Du Comportement
Int. J. Speleol. 4 (1972), pp. 139-169. Etude comparee du comportement alimentaire de deux poissons cavernicoles (Anoptichthys jordani Hubbs et Innes et Caecobarbus geertsi Blgr.l par Georges THINES et Nicole WISSOCQ* INTRODUCTION Dans un travail preliminaire pam dans ce meme Journal, Thines, Soffie et Vandenbussche (1966) avaient procede a l'analyse du comportement alimentaire du poisson cavernicole Anoptichthys Gen. et des hybrides F 1 obtenus par croisement Astyanax x Anoptichthys, la premiere espece etant la forme ancestrale epigee de la seconde. Les resultats de ces recherches avaient mis en evidence les traits essentiels de la reaction de la forme cavernicole Anoptichthys a la stimulation chimique. Ces donnees avaient egalement permis de caracteriser la stereotypie d'exploration de ce poisson consecutive a la stimulation chimique initiale. Les auteurs precites avaient etabli que l'exploration alimentaire s'effectuait sous la forme d'une plongee vers Ie fond suivie d'une recherche active localisee autour du point d'impact de la nourriture sur Ie substrat. L'efficacite de saisie, comparee a celIe de la forme ancestrale Astyanax, est tres deficitaire. De plus, la plongee vers Ie fond peut etre declenchee par des stimuli perturbants de nature mecanique. L'ensemble de ces observations avait ete soumis a la discussion et avait amene a conclure que Ie comportement alimentaire, outre les deficits evidents qu'il accuse sur Ie plan de l'efficacite spatio-temporelle de saisie, intervenait a titre substitutif dans des situations declenchant des comportements phobiques typiques chez la forme epigee Astyanax. POSITION DU PROBLEME Si I'on se refere a la classification de Vandel (1964), l'Anoptichthys est un cavernicole recent, tandis que Ie Caecobarbus est un cavernicole ancien. -

FLORIDA SOUTHEAST CONNECTION PROJECT RESOURCE REPORT 3 Fish, Wildlife, and Vegetation September 2014
FLORIDA SOUTHEAST CONNECTION PROJECT RESOURCE REPORT 3 Fish, Wildlife, and Vegetation September 2014 TABLE OF CONTENTS 3.0 RESOURCE REPORT 3 – FISH, WILDLIFE, AND VEGETATION .............................................. 3-1 3.1 INTRODUCTION ............................................................................................................................. 3-1 3.2 FISHERY RESOURCES ................................................................................................................... 3-1 3.2.1 Fisheries Habitat Classification .......................................................................................... 3-2 3.2.2 Existing Fishery Resources ................................................................................................ 3-2 3.2.3 Fisheries of Special Concern ............................................................................................. 3-2 3.2.4 Fisheries Impacts and Mitigation ........................................................................................ 3-2 3.2.4.1 Waterbody Construction Methods .................................................................................. 3-3 3.2.4.2 Vegetation Clearing ........................................................................................................ 3-3 3.2.4.3 Hydrostatic Test Water ................................................................................................... 3-4 3.2.4.4 Spill Prevention Control ................................................................................................. -
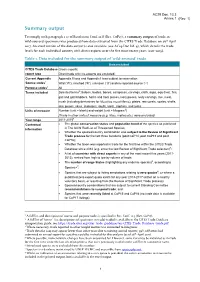
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g.