Spiny Lobster Growth Band Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
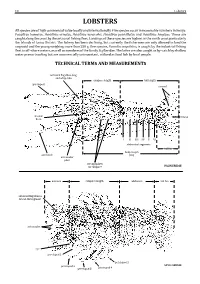
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

A Time Series of California Spiny Lobster (Panulirus Interruptus) Phyllosoma from 1951 to 2008 Links Abundance to Warm Oceanogr
KOSLOW ET AL.: LOBSTER PHYLLOSOMA ABUNDANCE LINKED TO WARM CONDITIONS CalCOFI Rep., Vol. 53, 2012 A TIME SERIES OF CALIFORNIA SPINY LOBSTER (PANULIRUS INTERRUPTUS) PHYLLOSOMA FROM 1951 TO 2008 LINKS ABUNDANCE TO WARM OCEANOGRAPHIC CONDITIONS IN SOUTHERN CALIFORNIA J. ANTHONY KOSLOW LauRA ROGERS-BENNETT DOUGLAS J. NEILSON Scripps Institution of Oceanography California Department of Fish and Game California Department of Fish and Game University of California, S.D. Bodega Marine Laboratory 4949 Viewridge Avenue La Jolla, CA 92093-0218 UC Davis, 2099 Westside Rd. San Diego, CA 92123 ph: (858) 534-7284 Bodega Bay, CA 94923-0247 [email protected] ABSTRACT The California spiny lobster (Panulirus interruptus) population is the basis for a valuable commercial and recreational fishery off southern California, yet little is known about its population dynamics. Studies based on CalCOFI sampling in the 1950s indicated that the abun- dance of phyllosoma larvae may be sensitive to ocean- ographic conditions such as El Niño events. To further study the potential influence of environmental variabil- ity and the fishery on lobster productivity, we developed a 60-year time series of the abundance of lobster phyl- losoma from the historical CalCOFI sample collection. Phyllosoma were removed from the midsummer cruises when the early-stage larvae are most abundant in the plankton nearshore. We found that the abundance of the early-stage phyllosoma displayed considerable inter- annual variability but was significantly positively corre- Figure 1. Commercial (solid circles), recreational (open triangles), and total lated with El Niño events, mean sea-surface temperature, landings (solid line) of spiny lobster off southern California. -

On the Vaccination of Shrimp Against White Spot Syndrome Virus
On the vaccination of shrimp against white spot syndrome virus Jeroen Witteveldt Promotoren: Prof. dr. J. M. Vlak Persoonlijk Hoogleraar bij de Leerstoelgroep Virologie Prof. dr. R. W. Goldbach Hoogleraar in de Virologie Co-promotor Dr. ir. M. C. W. van Hulten (Wetenschappelijk medewerker CSIRO, Brisbane, Australia) Promotiecommissie Prof. dr. P. Sorgeloos (Universiteit Gent, België) Prof. dr. J. A. J. Verreth (Wageningen Universiteit) Prof. dr. ir. H. F. J. Savelkoul (Wageningen Universiteit) Dr. ir. J. T. M. Koumans (Intervet International, Boxmeer, Nederland) Dit onderzoek werd uitgevoerd binnnen de onderzoekschool ‘Production Ecology and Resource Conservation’ (PE&RC) On the vaccination of shrimp against white spot syndrome virus Jeroen Witteveldt Proefschrift ter verkrijging van de graad van doctor op het gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. M. J. Kropff, in het openbaar te verdedigen op vrijdag 6 januari 2006 des namiddags te vier uur in de Aula Jeroen Witteveldt (2006) On the vaccination of shrimp against white spot syndrome virus Thesis Wageningen University – with references – with summary in Dutch ISBN: 90-8504-331-X Subject headings: WSSV, vaccination, immunology, Nimaviridae, Penaeus monodon CONTENTS Chapter 1 General introduction 1 Chapter 2 Nucleocapsid protein VP15 is the basic DNA binding protein of 17 white spot syndrome virus of shrimp Chapter 3 White spot syndrome virus envelope protein VP28 is involved in the 31 systemic infection of shrimp Chapter 4 Re-assessment of the neutralization -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Magnetic Remanence in the Western Atlantic Spiny Lobster, Panulirus Argus
J. exp. Biol. 113, 29-41 (1984) 29 Printed in Great Britain © The Company of Biologists Limited 1984 MAGNETIC REMANENCE IN THE WESTERN ATLANTIC SPINY LOBSTER, PANULIRUS ARGUS BY KENNETH J. LOHMANN* Department of Zoology, University of Florida, Gainesville, FL 32611, U.SA. and C. V. Whitney Laboratory for Experimental Marine Biology and Medicine, University of Florida, Route 1, Box 121, St Augustine, FL 32086, U.SA. Accepted 3 May 1984 SUMMARY The magnetic characteristics of 15 western Atlantic spiny lobsters (Panulirus argus) were analysed with a superconducting cryogenic magnetometer. Each specimen possessed a significant natural remanent magnetization (NRM) and isothermal remanent magnetization (IRM), in- dicating that ferromagnetic material is present. Analyses of the distribution of total remanence and mass-specific remanence indicate that magnetic material is concentrated in the cephalothorax, particularly in tissue associated with the fused thoracic ganglia. Mass-specific remanence and the total quantity of magnetic material in the cephalothorax and abdomen both increase as functions of carapace length. The NRM is significantly orientated in at least four regions of the body. The NRM of the left half of the posterior cephalothorax is directed pos- teriorly, while that of the right half is orientated anteriorly. In addition, the NRM of the middle cephalothorax is orientated toward the right side of the animal; the NRM of the telson-uropods region is directed toward the left. The functional significance of these regions of orientated remanence is not known, but such a pattern could result from the ordered alignment of per- manently magnetic particles comprising a magnetoreceptor system. INTRODUCTION The magnetic field of the earth influences the orientation of organisms ranging from unicellular algae (Lins de Barros, Esquivel, Danon & De Oliveira, 1981) and bacteria (Blakemore, 1975; Blakemore, Frankel & Kalmijn, 1980) to birds (e.g. -

Disease of Aquatic Organisms 100:89
Vol. 100: 89–93, 2012 DISEASES OF AQUATIC ORGANISMS Published August 27 doi: 10.3354/dao02510 Dis Aquat Org OPENPEN ACCESSCCESS INTRODUCTION Disease effects on lobster fisheries, ecology, and culture: overview of DAO Special 6 Donald C. Behringer1,2,*, Mark J. Butler IV3, Grant D. Stentiford4 1Program in Fisheries and Aquatic Sciences, School of Forest Resources and Conservation, University of Florida, Gainesville, Florida 32653, USA 2Emerging Pathogens Institute, University of Florida, Gainesville, Florida 32610, USA 3Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529, USA 4European Union Reference Laboratory for Crustacean Diseases, Centre for Environment, Fisheries and Aquaculture Science (Cefas), Weymouth Laboratory, Weymouth, Dorset DT4 8UB, UK ABSTRACT: Lobsters are prized by commercial and recreational fishermen worldwide, and their populations are therefore buffeted by fishery practices. But lobsters also remain integral members of their benthic communities where predator−prey relationships, competitive interactions, and host−pathogen dynamics push and pull at their population dynamics. Although lobsters have few reported pathogens and parasites relative to other decapod crustaceans, the rise of diseases with consequences for lobster fisheries and aquaculture has spotlighted the importance of disease for lobster biology, population dynamics and ecology. Researchers, managers, and fishers thus increasingly recognize the need to understand lobster pathogens and parasites so they can be managed proactively and their impacts minimized where possible. At the 2011 International Con- ference and Workshop on Lobster Biology and Management a special session on lobster diseases was convened and this special issue of Diseases of Aquatic Organisms highlights those proceed- ings with a suite of articles focused on diseases discussed during that session. -

California Spiny Lobster Scientific Name: Panulirus Interruptus Range
Fishery-at-a-Glance: California Spiny Lobster Scientific Name: Panulirus interruptus Range: Spiny Lobster range from Monterey, California southward to at least as far as Magdalena Bay, Baja California. The physical center of the range is within Mexico, and population density and fishery productivity is highest in this area. Habitat: As juveniles (less than 3 years of age), Spiny Lobster live in coastal rubble beds, but as adults, they are found on hard bottomed or rocky-reef habitat kelp forests. Size (length and weight): Adult Spiny Lobsters average 2 pounds in weight and about 12 inches total length, with males slightly larger than females. Adults more than 5 pounds are currently considered trophy individuals, although records exist from a century ago of 26 pound, 3 foot long lobsters. Life span: Spiny Lobsters can live up to 30 to 50 years. Reproduction: Spiny Lobsters mature at about 5 years of age, or 2.5-inch carapace length. They have a complex, 2-year reproductive cycle from mating to the settlement of juvenile lobsters. Fecundity increases with size, and females produce one brood of eggs per year. Prey: Spiny Lobsters are omnivorous, and act as important keystone predators within the southern California nearshore ecosystem. Adults forage at night for algae, fish, and many marine invertebrates. Predators: Predators of juvenile Spiny Lobsters include California Sheephead, Cabezon, rockfishes, Kelp Bass, Giant Sea Bass, and octopus. Predators of adult lobsters tend to be the larger individuals such as male California Sheephead and Giant Sea Bass. Fishery: The commercial fishery accounted for approximately 312 metric tons (688,000 lb) in ex- vessel landings and $12.7 million in ex-vessel value during the 2017-2018 fishing season. -

A New Pathogenic Virus in the Caribbean Spiny Lobster Panulirus Argus from the Florida Keys Jeffrey D
Old Dominion University ODU Digital Commons Biological Sciences Faculty Publications Biological Sciences 2004 A New Pathogenic Virus in the Caribbean Spiny Lobster Panulirus argus from the Florida Keys Jeffrey D. Shields Donald C. Behringer Jr. Old Dominion University Follow this and additional works at: https://digitalcommons.odu.edu/biology_fac_pubs Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Repository Citation Shields, Jeffrey D. and Behringer, Donald C. Jr., "A New Pathogenic Virus in the Caribbean Spiny Lobster Panulirus argus from the Florida Keys" (2004). Biological Sciences Faculty Publications. 323. https://digitalcommons.odu.edu/biology_fac_pubs/323 Original Publication Citation Shields, J. D., & Behringer, D. C. (2004). A new pathogenic virus in the Caribbean spiny lobster panulirus argus from the Florida Keys. Diseases of Aquatic Organisms, 59(2), 109-118. doi:10.3354/dao059109 This Article is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Faculty Publications by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. DISEASES OF AQUATIC ORGANISMS Vol. 59: 109–118, 2004 Published May 5 Dis Aquat Org A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys Jeffrey D. Shields1,*, Donald C. Behringer Jr2 1Virginia Institute of Marine Science, The College of William & Mary, Gloucester Point, Virginia 23062, USA 2Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529, USA ABSTRACT: A pathogenic virus was diagnosed from juvenile Caribbean spiny lobsters Panulirus argus from the Florida Keys. -

Caribbean Spiny Lobster, Panulirus Argus1 Christian L
FA147 Candidate Species for Florida Aquaculture: Caribbean Spiny Lobster, Panulirus argus1 Christian L. Miller, Cortney L. Ohs, and R. LeRoy Creswell2 Caribbean Spiny Lobster General Description The Caribbean Spiny Lobster, Panulirus argus, is a member of the family of spiny lobsters, Palinuridae. The animal has a cylindrical carapace, which is covered with forward- projecting spines, and two prominent rostral horns which extend over the eyes. The most recognizable characteristic of the species is the pair of long, whip-like antennae, which are covered in short 1–2 mm spines. The tail is smooth, and the tail fan is composed of a central telson bordered by a pair of uropods on either side. Adult coloration varies from a green and brown to deep red and black dorsal carapace Figure 1. Illustration reproduced with permission of FAO. FAO Species and tail with light gray to tan sides and ventral surface. Identification Sheets, Area 31—West Central Atlantic (Volume 5). Individuals also have pronounced white to yellow ocelli on Credits: FAO, Rome, Italy. 1978 the second and sixth tail segments. Driven by high market value and limited availability, the development of both experimental and commercial- The spiny lobster inhabits coastal waters and shallow scale spiny lobster aquaculture operations are ongoing continental shelf waters of the Atlantic seaboard and por- worldwide. There has been considerable interest in tions of the Gulf of Mexico. Its range extends from North developing culture technologies for the 47 species (Lipcius Carolina southward to Brazil, including Bermuda and the and Eggleston 2000) in this family. Past studies focusing Gulf of Mexico (Marx and Herrnkind 1986). -

Panulirus Argus (Caribbean Spiny Lobster)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Panulirus argus (Caribbean Spiny Lobster) Order: Decapoda (Shrimps, Lobsters and Crabs) Class: Malacostraca (Crustaceans: Shrimps, Sand-hoppers and Woodlice) Phylum: Arthropoda (Arthropods) Fig. 1. Caribbean spiny lobster, Panulirus argus. [http://www.bbc.co.uk/nature/13780718, downloaded 18 March 2015] TRAITS. Panulirus argus has a carapace of a sub-cylindrical shape which has numerous long front-facing spines, and rostral horns point forward over the stalked compound eyes located at the front of the head (Crawford and De Smidt, 1922) (Fig. 1). Their characteristic whip-like antennae project from the head and are lined with setae (hairs) and spines, and the antennules are approximately two thirds of the carapace length. The abdomen has notches along the lateral margins but the rest is smooth and flat (Holthuis, 1991). The body colour can range from tan or grey, spotted with brown, black, green, red or purple spots (Crawford and De Smidt, 1922). The legs are striped in a dull blue colour, they have orange or black pleopods (swimming legs) (Calinski and Lyons, 1983). In juveniles, a wide white line follows the middle of the purplish body (Eldred et al., 1972; Kanciruk, 1980). Adult lobsters have white, yellow or brown stripes on the tail fans (Seudeal, 2013). Spiny lobsters are sexually dimorphic (Marx and Herrnkind, 1986). Males tend to have longer carapaces; the sternum is broader than in females and it has less to none of the striations found on females (Marx and Herrnkind, 1986). Males have raised genital openings on the base of the abdomen and females have gonopores (Marx and Herrnkind, 1986). -

Patterns of Spiny Lobster (Panulirus Argus) Postlarval Recruitment in the Caribbean: a CRTR Project
Patterns of Spiny Lobster (Panulirus argus) Postlarval Recruitment in the Caribbean: A CRTR Project MARK J. BUTLER 1, ANGELA M. MOJICA 1, ELOY SOSA-CORDERO 2, MARINES MILLET 3, PAUL SANCHEZ- NAVARRO 4, MIGUEL A. MALDONADO 4, JUAN POSADA 5, BLADIMIR RODRIGUEZ 6, CARLOS M. RIVAS 6, ADRIAN OVIEDO 4, MARCIO ARRONE 4, MARTHA PRADA 7, NICK BACH 8, NILDA JIMENEZ 9, MARIA DEL CARMEN GARCIA-RIVAS 10, KIRAH FORMAN11, DONALD C. BEHRINGER, JR. 12, THOMAS MATTHEWS 13, CLAIRE PARIS 14, and ROBERT COWEN 14 1Department Biological Sciences, Old Dominion University, Norfolk, Virginia 23529 USA, 2ECOSUR, Chetumal, Mexico, 3 Cozumel National Park, Cozumel, Mexico, 4Center Ecology Akumal, Akumal, Mexico, 5University Simon Bolivar, Caracas, Venezuela, 6Fundación Científica Los Roques, Los Roques, Venezuela, 7Blue Dream Ltd., San Andres Islands, Colombia, 8Roatan Marine Park, Roatan, Honduras, 9Department of Natural Resources, Puerto Rico, 10Reserva de la Biosfera Banco Chinchorro y Parque de Arrecifes de Xcalac, Chetumal, Mexico, 11Belize Department of Fisheries, Hol Chan Marine Reserve, Belize City, Belize, 12Program in Fisheries and Aquatic Sciences, University of Florida, Gainesville, Florida USA, 13Fish and Wildlife Conservation Commission, Marathon, Florida USA, 14Rosenstiel School of Marine and Atmospheric Sciences, University of Miami, Miami, Florida USA ABSTRACT As part of the Coral Reef Targeted Research (CRTR) Program, a partnership between the Global Environment Facility and the World Bank, our research team examined the recruitment patterns of Caribbean spiny lobster (Panulirus argus) postlarvae among regions in the Caribbean, with a particular focus on Mesoamerica. Our goal was to collect comparable information on postlarval supply among regions and to provide data to test predictions of connectivity generated from a coupled biophysical oceanographic model of lobster larval dispersal.