California Spiny Lobster (Panulirus Interruptus) Movement Behavior and Habitat Use: Implications for the Effectiveness of Marine Protected Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

American Lobster Settlement Index |Update 2019
American Lobster Settlement Index | Update 2019 Compiled by: R. Wahle and K. Holmes Participants: ME DMR (K. Reardon, R. Russell), MA DMF (T. Pugh, K. Whitmore), C. Brown (Ready Seafood Co.), J. Drouin (Little River Lobster Co.), RI DFW (S. Olszewski, C. McManus), NH F&G (J. Carloni), DFO Canada (A. Rondeau, N. Asselin, J. Gaudette, P. Lawton, S. Armsworthy, A. Cook), UNB, St. John (R. Rochette), PEIFA (L. Ramsay, M. Giffen), PEI DAF (R. MacMillan), Fishermen & Scientists Research Society (S. Scott-Tibbets), Memorial University (A. Le Bris), http://umaine.edu/wahlelab/current-projects/american-lobster-settlement-index/ Several months now into the COVID-19 pandemic, we can only look back to the 2019 fishing year with some nostalgia. In calendar year 2019, Canadian lobster landings continued to boast near all-time highs. And while US landings have slipped a bit from their own historic highs, much of those losses have been offset by continued high value. That is, until now. All that changed almost overnight with the onset of the pandemic, as demand for lobster plummeted with shuttered restaurants and overseas commerce, dragging down the price of lobster, much to the distress of fishing communities up and down the coast. As we enter the 2020 summer fishing season, the lobster industry grapples to find a new normal as it accommodates health protocols, readjusts markets and scales back revenue expectations. In kind, state and federal marine resource monitoring programs are equally rethinking sampling programs under shrinking tax revenues and new safety standards. Last year’s Update examined how well ALSI predicted trends in the fishery from Fundy to Rhode Island. -

Metanephrops Challengeri)
Population genetics of New Zealand Scampi (Metanephrops challengeri) Alexander Verry A thesis submitted to Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity. Victoria University of Wellington 2017 Page | I Abstract A fundamental goal of fisheries management is sustainable harvesting and the preservation of properly functioning populations. Therefore, an important aspect of management is the identification of demographically independent populations (stocks), which is achieved by estimating the movement of individuals between areas. A range of methods have been developed to determine the level of connectivity among populations; some measure this directly (e.g. mark- recapture) while others use indirect measures (e.g. population genetics). Each species presents a different set of challenges for methods that estimate levels of connectivity. Metanephrops challengeri is a species of nephropid lobster that supports a commercial fishery and inhabits the continental shelf and slope of New Zealand. Very little research on population structure has been reported for this species and it presents a unique set of challenges compared to finfish species. M. challengeri have a short pelagic larval duration lasting up to five days which limits the dispersal potential of larvae, potentially leading to low levels of connectivity among populations. The aim of this study was to examine the genetic population structure of the New Zealand M. challengeri fishery. DNA was extracted from M. challengeri samples collected from the eastern coast of the North Island (from the Bay of Plenty to the Wairarapa), the Chatham Rise, and near the Auckland Islands. DNA from the mitochondrial CO1 gene and nuclear ITS-1 region was amplified and sequenced. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

B Chromosomes, Ribosomal Genes and Telomeric Sequences
Genetica DOI 10.1007/s10709-012-9691-4 Comparative cytogenetics in four species of Palinuridae: B chromosomes, ribosomal genes and telomeric sequences Susanna Salvadori • Elisabetta Coluccia • Federica Deidda • Angelo Cau • Rita Cannas • Anna Maria Deiana Received: 18 May 2012 / Accepted: 20 November 2012 Ó Springer Science+Business Media Dordrecht 2012 Abstract The evolutionary pathway of Palinuridae Introduction (Crustacea, Decapoda) is still controversial, uncertain and unexplored, expecially from a karyological point of view. Scyllaridae and Palinuridae constitute the Achelata group, Here we describe the South African spiny lobster Jasus considered to be monophyletic and the Palinuridae now lalandii karyotype: n and 2n values, heterochromatin dis- includes the Sinaxidae family (Patek et al. 2006; George tribution, nucleolar organizer region (NOR) location and 2006; Groeneweld et al. 2007; Palero et al. 2009a; Tsang telomeric repeat structure and location. To compare the et al. 2009). genomic and chromosomal organization in Palinuridae we Fossil records from North America, Europe and Aus- located NORs in Panulirus regius, Palinurus gilchristi and tralia suggest that the Palinuridae arose in the early Palinurus mauritanicus: all species showed multiple Mesozoic (George 2006). The family currently comprises NORs. In J. lalandii NORs were located on three chro- approximately ten genera and fifty species (including those mosome pairs, with interindividual polymorphism. In of the Synaxidae), which can be subdivided into two P. regius andinthetwoPalinurus species NORs were located groups: the Stridentes, and the Silentes. This family has on two chromosome pairs. In the two last species 45S received much attention and there are data on comparative ribosomal gene loci were also found on B chromosomes. -

The World Lobster Market
GLOBEFISH RESEARCH PROGRAMME The world lobster market Volume 123 GRP123coverB5.indd 1 23/01/2017 15:06:37 FAO GLOBEFISH RESEARCH PROGRAMME VOL. 123 The world lobster market by Graciela Pereira Helga Josupeit FAO Consultants Products, Trade and Marketing Branch Fisheries and Aquaculture Policy and Resources Division Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2017 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109631-4 © FAO, 2017 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
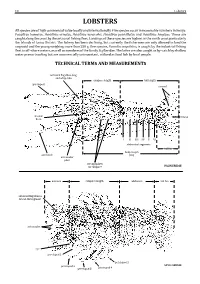
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance
Decapod crustacean grooming: Functional morphology, adaptive value, and phylogenetic significance N RAYMOND T.BAUER Center for Crustacean Research, University of Southwestern Louisiana, USA ABSTRACT Grooming behavior is well developed in many decapod crustaceans. Antennular grooming by the third maxillipedes is found throughout the Decapoda. Gill cleaning mechanisms are qaite variable: chelipede brushes, setiferous epipods, epipod-setobranch systems. However, microstructure of gill cleaning setae, which are equipped with digitate scale setules, is quite conservative. General body grooming, performed by serrate setal brushes on chelipedes and/or posterior pereiopods, is best developed in decapods at a natant grade of body morphology. Brachyuran crabs exhibit less body grooming and virtually no specialized body grooming structures. It is hypothesized that the fouling pressures for body grooming are more severe in natant than in replant decapods. Epizoic fouling, particularly microbial fouling, and sediment fouling have been shown r I m ans of amputation experiments to produce severe effects on olfactory hairs, gills, and i.icubated embryos within short lime periods. Grooming has been strongly suggested as an important factor in the coevolution of a rhizocephalan parasite and its anomuran host. The behavioral organization of grooming is poorly studied; the nature of stimuli promoting grooming is not understood. Grooming characters may contribute to an understanding of certain aspects of decapod phylogeny. The occurrence of specialized antennal grooming brushes in the Stenopodidea, Caridea, and Dendrobranchiata is probably not due to convergence; alternative hypotheses are proposed to explain the distribution of this grooming character. Gill cleaning and general body grooming characters support a thalassinidean origin of the Anomura; the hypothesis of brachyuran monophyly is supported by the conservative and unique gill-cleaning method of the group. -

A Synthesis of Existing Data on Larval Rock Lobster Distribution in Southern Australia Project 96/107
A synthesis of existing data on larval rock lobster distribution in southern Australia Project 96/107 DEVELOPMENT CORPORATION Final Report to FRDC CS I RO Final Report the Project 96/107 B. Bruce, R. Bradford, 0. Griffin, C. Gardner and J. Young CSIRO Marine Laboratories Hobart ISBN: 0 643 062270 FRDC FIN.AL REPORT: PROJECT NO. 96/107 3, 5. 5.1 Scope and Rationale of 5.2 Sources of data {collaborating institutions) ......................................................................... 10 5.3 Sample Coverage ............................................................................................................... i2 5.3.1 Geographic ................................................................................................................................................. 12 5.3.2 Annual ........................................................................................................................................................ 13 5.3.3 Seasonal .................................................................................................................................................... 13 5.4 Net Systems: ...................................................................................................................... 14 5.4.1 Surface sampling ........................................................................................................................................ 14 Surface net ..................................................................................................................................... -

A Time Series of California Spiny Lobster (Panulirus Interruptus) Phyllosoma from 1951 to 2008 Links Abundance to Warm Oceanogr
KOSLOW ET AL.: LOBSTER PHYLLOSOMA ABUNDANCE LINKED TO WARM CONDITIONS CalCOFI Rep., Vol. 53, 2012 A TIME SERIES OF CALIFORNIA SPINY LOBSTER (PANULIRUS INTERRUPTUS) PHYLLOSOMA FROM 1951 TO 2008 LINKS ABUNDANCE TO WARM OCEANOGRAPHIC CONDITIONS IN SOUTHERN CALIFORNIA J. ANTHONY KOSLOW LauRA ROGERS-BENNETT DOUGLAS J. NEILSON Scripps Institution of Oceanography California Department of Fish and Game California Department of Fish and Game University of California, S.D. Bodega Marine Laboratory 4949 Viewridge Avenue La Jolla, CA 92093-0218 UC Davis, 2099 Westside Rd. San Diego, CA 92123 ph: (858) 534-7284 Bodega Bay, CA 94923-0247 [email protected] ABSTRACT The California spiny lobster (Panulirus interruptus) population is the basis for a valuable commercial and recreational fishery off southern California, yet little is known about its population dynamics. Studies based on CalCOFI sampling in the 1950s indicated that the abun- dance of phyllosoma larvae may be sensitive to ocean- ographic conditions such as El Niño events. To further study the potential influence of environmental variabil- ity and the fishery on lobster productivity, we developed a 60-year time series of the abundance of lobster phyl- losoma from the historical CalCOFI sample collection. Phyllosoma were removed from the midsummer cruises when the early-stage larvae are most abundant in the plankton nearshore. We found that the abundance of the early-stage phyllosoma displayed considerable inter- annual variability but was significantly positively corre- Figure 1. Commercial (solid circles), recreational (open triangles), and total lated with El Niño events, mean sea-surface temperature, landings (solid line) of spiny lobster off southern California. -

And Red Sea Urchins
NEGATIVELY CORRELATED ABUNDANCE SUGGESTS COMPETITION BETWEEN RED ABALONE (Haliotis rufescens) AND RED SEA URCHINS (Mesocentrotus franciscanus) INSIDE AND OUTSIDE ESTABLISHED MPAs CLOSED TO COMMERCIAL SEA URCHIN HARVEST IN NORTHERN CALIFORNIA By Johnathan Centoni A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment of the Requirements for the Degree Master of Science in Biology Committee Membership Dr. Sean Craig, Committee Chair Dr. Brian Tissot, Committee Member Dr. Paul Bourdeau, Committee Member Dr. Joe Tyburczy, Committee Member Dr. Erik Jules, Program Graduate Coordinator May 2018 ABSTRACT NEGATIVELY CORRELATED ABUNDANCE SUGGESTS COMPETITION BETWEEN RED ABALONE (Haliotis rufescens) AND RED SEA URCHINS (Mesocentrotus franciscanus) INSIDE AND OUTSIDE ESTABLISHED MPAs CLOSED TO COMMERCIAL SEA URCHIN HARVEST IN NORTHERN CALIFORNIA Johnathan Centoni Red abalone and sea urchins are both important herbivores that potentially compete with each other for resources like food and space along the California coast. Red abalone supported a socioeconomically important recreational fishery during this study (which was closed in 2018) and red sea urchins support an important commercial fishery. Both red sea urchins and red abalone feed on the same macroalgae (including Pterygophora californica, Laminaria setchellii, Stephanocystis osmundacea, Costaria costata, Alaria marginata, Nereocystis leutkeana), and a low abundance of this food source during the period of this project may have created a highly competitive environment for urchins and abalone. Evidence that suggests competition between red abalone and red sea urchins can be seen within data collected during the years of this study (2014-2016): a significantly higher red sea urchin density, concomitant with a significantly lower red abalone density, was observed within areas closed to commercial sea urchin harvest (in MPAs) compared to nearby reference areas open to sea urchin harvest.