(Jasus Edwardsii Hutton, 1875) Larvae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Metanephrops Challengeri)
Population genetics of New Zealand Scampi (Metanephrops challengeri) Alexander Verry A thesis submitted to Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity. Victoria University of Wellington 2017 Page | I Abstract A fundamental goal of fisheries management is sustainable harvesting and the preservation of properly functioning populations. Therefore, an important aspect of management is the identification of demographically independent populations (stocks), which is achieved by estimating the movement of individuals between areas. A range of methods have been developed to determine the level of connectivity among populations; some measure this directly (e.g. mark- recapture) while others use indirect measures (e.g. population genetics). Each species presents a different set of challenges for methods that estimate levels of connectivity. Metanephrops challengeri is a species of nephropid lobster that supports a commercial fishery and inhabits the continental shelf and slope of New Zealand. Very little research on population structure has been reported for this species and it presents a unique set of challenges compared to finfish species. M. challengeri have a short pelagic larval duration lasting up to five days which limits the dispersal potential of larvae, potentially leading to low levels of connectivity among populations. The aim of this study was to examine the genetic population structure of the New Zealand M. challengeri fishery. DNA was extracted from M. challengeri samples collected from the eastern coast of the North Island (from the Bay of Plenty to the Wairarapa), the Chatham Rise, and near the Auckland Islands. DNA from the mitochondrial CO1 gene and nuclear ITS-1 region was amplified and sequenced. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

The World Lobster Market
GLOBEFISH RESEARCH PROGRAMME The world lobster market Volume 123 GRP123coverB5.indd 1 23/01/2017 15:06:37 FAO GLOBEFISH RESEARCH PROGRAMME VOL. 123 The world lobster market by Graciela Pereira Helga Josupeit FAO Consultants Products, Trade and Marketing Branch Fisheries and Aquaculture Policy and Resources Division Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2017 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109631-4 © FAO, 2017 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
Lobsters LOBSTERS§
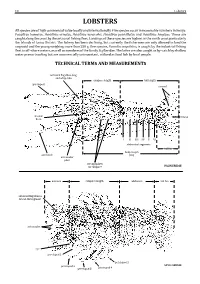
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

12 REVISED J Caveorum Profile
Document SPRFMO-III-SWG-12 Information describing Jasus caveorum fisheries relating to the South Pacific Regional Fisheries Management Organisation REVISED 20 February 2007 DRAFT 1. Overview.......................................................................................................................2 2. Taxonomy.....................................................................................................................3 2.1 Phylum..................................................................................................................3 2.2 Class.....................................................................................................................3 2.3 Order.....................................................................................................................3 2.4 Family...................................................................................................................3 2.5 Genus and species.................................................................................................3 2.6 Scientific synonyms...............................................................................................3 2.7 Common names.....................................................................................................3 2.8 Molecular (DNA or biochemical) bar coding.........................................................3 3. Species characteristics....................................................................................................3 3.1 Global distribution -

A Synthesis of Existing Data on Larval Rock Lobster Distribution in Southern Australia Project 96/107
A synthesis of existing data on larval rock lobster distribution in southern Australia Project 96/107 DEVELOPMENT CORPORATION Final Report to FRDC CS I RO Final Report the Project 96/107 B. Bruce, R. Bradford, 0. Griffin, C. Gardner and J. Young CSIRO Marine Laboratories Hobart ISBN: 0 643 062270 FRDC FIN.AL REPORT: PROJECT NO. 96/107 3, 5. 5.1 Scope and Rationale of 5.2 Sources of data {collaborating institutions) ......................................................................... 10 5.3 Sample Coverage ............................................................................................................... i2 5.3.1 Geographic ................................................................................................................................................. 12 5.3.2 Annual ........................................................................................................................................................ 13 5.3.3 Seasonal .................................................................................................................................................... 13 5.4 Net Systems: ...................................................................................................................... 14 5.4.1 Surface sampling ........................................................................................................................................ 14 Surface net ..................................................................................................................................... -

Evaluation of Genes Involved in Norway Lobster (Nephrops Norvegicus) Female Sexual Maturation Using Transcriptomic Analysis
Hydrobiologia https://doi.org/10.1007/s10750-018-3521-3 CRUSTACEAN GENOMICS Evaluation of genes involved in Norway lobster (Nephrops norvegicus) female sexual maturation using transcriptomic analysis Guiomar Rotllant . Tuan Viet Nguyen . David Hurwood . Valerio Sbragaglia . Tomer Ventura . Joan B. Company . Silvia Joly . Abigail Elizur . Peter B. Mather Received: 6 October 2017 / Revised: 17 January 2018 / Accepted: 20 January 2018 Ó Springer International Publishing AG, part of Springer Nature 2018 Abstract The Norway lobster Nephrops norvegicus technology applied to multiple tissues. Ovarian mat- is the most important commercial crustacean species uration-related differential expression patterns were in Europe. Recent decline in wild captures and a observed for 4362 transcripts in ovary, hepatopan- reduction in total abundance and size at first matura- creas, eyestalk, brain, and thoracic ganglia in N. tion indicate that the species is overexploited. Increas- norvegicus. Transcripts detected in the study include ing knowledge of its reproduction, specifically at the vitellogenin, crustacean hyperglycaemic hormone, molecular level will be mandatory to improving retinoid X receptor, heat shock protein 90 and proteins fisheries management. The current study investigated encoding lipid and carbohydrate metabolizing differences between immature and mature N. norvegi- enzymes. From the study, data were collected that cus females using Next Generation Sequencing can prove valuable in developing more comprehensive knowledge of the reproductive system in this lobster species during the ovarian maturation process. Addi- Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10750-018-3521-3) con- tional studies will be required, however, to identify tains supplementary material, which is available to authorized potential novel genes and to develop a molecular users. -

Growth of Tissues Related to Haemolymph Copper Throughout the Moult Cycle of the Lobster Homarus Gammarus
MARINE ECOLOGY PROGRESS SERIES Vol. 247: 165–172, 2003 Published February 4 Mar Ecol Prog Ser Growth of tissues related to haemolymph copper throughout the moult cycle of the lobster Homarus gammarus Massimo Devescovi*, >edomil Lucu Rud-er Bo$kovi´c Institute, Center for Marine Research, G. Paliaga 5, 52210 Rovinj, Croatia ABSTRACT: The relationship between tissue weights and haemolymph copper levels in field- collected European lobsters Homarus gammarus was investigated to assess whether haemolymph copper concentration can be used to reliably determine nutritional condition in lobsters. During the moult cycle, the soft tissue weight (mostly muscle) increased concomitantly with a decrease in haemolymph weight while exoskeleton weight increased from postmoult to intermoult. Haemolymph copper concentration gradually increased from postmoult to premoult with a range of 29.7 (Stage B) to –1 163.5 µg g wet weight (ww) (Stage D0). Our measurements suggest that the main processes that in- crease haemolymph copper concentration is a reduction in haemolymph space and an increase in haemolymph copper content, indicating synthesis of new haemocyanin for the oxygen supply of grow- ing tissues. During the moult cycle, haemolymph copper content was proportional to soft tissue mass and amounted to 43.6 µg Cu g–1 of soft-tissue ww (95% confidence limits = 35.5 and 51.6 µg g–1). Models for estimating soft-tissue and haemolymph wet weights from the haemolymph copper con- centration and moult stage are presented. The use of haemolymph copper concentration as an index of nutritional condition reflecting tissue growth and food quantity and quality is validated. KEY WORDS: European lobster · Exoskeleton · Haemolymph · Muscle · Soft tissues · Haemocyanin · Copper requirement · Condition index Resale or republication not permitted without written consent of the publisher INTRODUCTION muscle weight is correlated to haemolymph protein concentration. -

A C-Type Lectin Highly Expressed in Portunus Trituberculatus Intestine Functions in AMP Regulation and Prophenoloxidase Activation
antibiotics Article A C-Type Lectin Highly Expressed in Portunus trituberculatus Intestine Functions in AMP Regulation and Prophenoloxidase Activation Yuan Liu 1,2,3,4,*, Yue Su 1,4, Ao Zhang 1 and Zhaoxia Cui 5 1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China; [email protected] (Y.S.); [email protected] (A.Z.) 2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China 3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China 4 University of Chinese Academy of Sciences, Beijing 100049, China 5 School of Marine Science, Ningbo University, Ningbo 315211, China; [email protected] * Correspondence: [email protected]; Tel.: +86-532-8289-8637 Abstract: A C-type lectin (PtCLec2) from Portunus trituberculatus was identified for characterization of its role in defense and innate immunity. PtCLec2 contains a single carbohydrate-recognition domain (CRD) with a conserved QPD motif, which was predicted to have galactose specificity. The mRNA expression of PtCLec2 was predominantly detected in intestine and increased rapidly and significantly upon pathogen challenge. The recombinant PtCLec2 (rPtCLec2) could bind various microorganisms and PAMPs with weak binding ability to yeast and PGN. It agglutinated the tested Gram-negative bacteria (Vibrio alginolyticus and Pseudomonas aeruginosa), Gram-positive bacteria Citation: Liu, Y.; Su, Y.; Zhang, A.; (Staphylococcus aureus and Micrococcus luteus), and rabbit erythrocytes in the presence of exogenous Cui, Z. A C-Type Lectin Highly Ca2+, and these agglutination activities were suppressed by LPS, D-galactose, and D-mannose. -

The Utilization of Lobsters by Humans in the Mediterranean Basin from the Prehistoric Era to the Modern Era – an Interdisciplinary Short Review
Athens Journal of Mediterranean Studies- Volume 1, Issue 3 – Pages 223-234 The Utilization of Lobsters by Humans in the Mediterranean Basin from the Prehistoric Era to the Modern Era – An Interdisciplinary Short Review By Ehud Spanier The Mediterranean and Red Seas host a variety of clawed, spiny and slipper lobsters. Lobsters' utilization in ancient times varied, ranging from complete prohibition by the Jewish religion, to that of epicurean status in the Roman world. One of the earliest known illustrations of a spiny lobster was a wall carving in Egypt depicting the Queen Hatshepsut expedition to the Red Sea in the 15th century BC. Lobsters were known by the ancient Greeks and Romans as was expressed in art forms and writings. Lobsters also appeared in ancient mosaics and coins. The writings of naturalists and philosophers from the Roman-Hellenistic period, together with illustrative records indicate that lobsters were a popular food and there was considerable knowledge of their classification, biology and fisheries. The popularity of lobsters as gourmet food increased with time followed by an expansion of the scientific knowledge as well as over exploitation of these resources. Keywords: Antiquity, Biology, Fisheries, Lobsters, Mediterranean. Introduction A variety of edible lobsters, that are still commercially significant to human inhabitants today, are found in the water of the Mediterranean and adjacent Red Sea regions. This important marine resource includes at least two Mediterranean clawed lobsters (the European lobster, Homarus gammarus, and the Norway lobster, Nephrops norvegicus), five spiny lobsters (two in the Mediterranean: the common spiny lobster, Palinurus elephas, and the pink spiny lobster, P.