Growth of Tissues Related to Haemolymph Copper Throughout the Moult Cycle of the Lobster Homarus Gammarus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
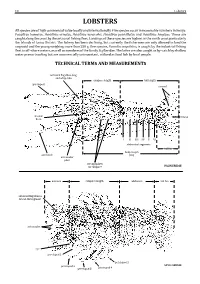
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

(Jasus Edwardsii Hutton, 1875) Larvae
Environmental Physiology of Cultured Early-Stage Southern Rock Lobster (Jasus edwardsii Hutton, 1875) Larvae Michel Francois Marie Bermudes Submitted in fulfilment of the requirements for the degree of Doctor Of Philosophy University of Tasmania November 2002 Declarations This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information in duly acknowledged in the thesis, and to the best of the candidate's knowledge and belief, no material previously published or written by another person except where due acknowledgment is made in the text of the thesis. Michel Francois Marie Bermudes This thesis may be available for loan and limited copying in accordance with the Copyright Act 1968. Michel Francois Marie Bermudes Abstract The aim of this project was to define more clearly the culture conditions for the propagation of the southern rock lobster (Jasus echvardsii) in relation to environmental bioenergetic constraints. The effects of temperature and photoperiod on the first three stages of development were first studied in small-scale culture experiments. Larvae reared at 18°C developed faster and reached a larger size at stage IV than larvae cultured at 14°C. Development through stage II was shorter under continuous light. However, the pattern of response to photoperiod shifted at stage III when growth was highest in all the light/dark phase treatments than under continuous light. The influence of temperature and light intensity in early-stage larvae was further investigated through behavioural and physiological studies. Results obtained in stages I, II and III larvae indicated an energetic imbalance at high temperature (-22°C). -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus Indicus and Scyllarides Haanii) from South of Java, Indonesia
HAYATI Journal of Biosciences 23 (2016) 101e105 HOSTED BY Contents lists available at ScienceDirect HAYATI Journal of Biosciences journal homepage: http://www.journals.elsevier.com/ hayati-journal-of-biosciences Original research article Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus indicus and Scyllarides haanii) From South of Java, Indonesia * Yusli Wardiatno, Agus Alim Hakim, Ali Mashar, Nurlisa Alias Butet, Luky Adrianto Department of Aquatic Resources Management, Faculty of Fisheries and Marine Science, Bogor Agricultural University, Bogor, West Java, Indonesia. article info abstract Article history: Two species of slipper lobster, Thenus indicus Leach, 1815, and Scyllarides haanii De Haan, 1841, are re- Received 5 February 2016 ported for the first time from the coastal waters of South of Java, part of the Indian Ocean. A total of two Received in revised form specimens, one specimen of T. indicus from Palabuhanratu Bay and one specimen of S. haanii from 30 April 2016 Yogyakarta coastal waters, were collected in April and September 2015, respectively. Descriptions and Accepted 9 May 2016 illustrations of the morphological characteristics of the two species and their habitat are presented. Available online 26 May 2016 Copyright © 2016 Institut Pertanian Bogor. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). KEYWORDS: Crustacean, Decapoda, first record, Indian Ocean, slipper lobster 1. Introduction only one species, Thenus orientalis. Taxonomic and biodiversity studies on Indonesian lobsters resulted in the collection of two From a fishery point of view, slipper lobsters of the family different species of family Scyllaridae from South of Java. -

Spiny Lobster (Panulirus Sp.) Life Cycle – a Review and Fisheries Management Implications
Research Notes… Spiny Lobster (Panulirus sp.) Life Cycle – a review and fisheries management implications Léo Barret Abstract The fishery for spiny lobster around the inner granitic islands of the Seychelles has been closed for two consecutive years (2017-2019). Declining trends in indicators of abundance and the loss of coral reef habitats led to the SeyCCAT funded lobster project, aimed at establishing a science-based restoration of commercially important spiny lobster habitats to help develop a sustainable fishery – a project partnership between the University of Seychelles (UniSey), the Seychelles Fishing Authority (SFA), and the Marine Conservation Society Seychelles (MCSS). Spiny lobsters have a complex mero-planktonic lifecycle from a larva living in the open sea to an adult living on the sea floor. The management of the fishery requires a good understanding on all the stages of the spiny lobster life cycle. In this context, the objective of this paper is to provide a short review of the spiny lobster (Panulirus sp.) life cycle and the ecological and fisheries management implications. Introduction (a Seychelles perspective) Spiny lobsters (Palinuridae) are one of the most valuable commercial seafood species globally; for example in Australia, lobster is the country’s most valuable fishery in terms of both overall production and value of export, with a Gross Value of Production of $610 million AUD in 2014 (Plagányi et al., 2018). The FAO annual catch data of spiny (Palinuridae) and clawed (Nephropidae) lobsters has been steadily increasing over the past few decades and the 2017 global catch estimate was 315,469 t (FAO, 2019). Unlike the global trend, Seychelles’ lobster fishery catch has decreased over the past decade, with some of the major challenges being limited recruitment, poaching, and rapid ecosystem change, such as the loss of coral reef habitats due to coral bleaching events. -

Homarus Americanus H
BioInvasions Records (2021) Volume 10, Issue 1: 170–180 CORRECTED PROOF Rapid Communication An American in the Aegean: first record of the American lobster Homarus americanus H. Milne Edwards, 1837 from the eastern Mediterranean Sea Thodoros E. Kampouris1,*, Georgios A. Gkafas2, Joanne Sarantopoulou2, Athanasios Exadactylos2 and Ioannis E. Batjakas1 1Marine Sciences Department, School of the Environment, University of the Aegean, University Hill, Mytilene, Lesvos Island, 81100, Greece 2Department of Ichthyology & Aquatic Environment, School of Agricultural Sciences, University of Thessaly, Fytoko Street, Volos, 38 445, Greece Author e-mails: [email protected] (TEK), [email protected] (IEB), [email protected] (GAG), [email protected] (JS), [email protected] (AE) *Corresponding author Citation: Kampouris TE, Gkafas GA, Sarantopoulou J, Exadactylos A, Batjakas Abstract IE (2021) An American in the Aegean: first record of the American lobster A male Homarus americanus individual, commonly known as the American lobster, Homarus americanus H. Milne Edwards, was caught by artisanal fishermen at Chalkidiki Peninsula, Greece, north-west Aegean 1837 from the eastern Mediterranean Sea. Sea on 26 August 2019. The individual weighted 628.1 g and measured 96.7 mm in BioInvasions Records 10(1): 170–180, carapace length (CL) and 31.44 cm in total length (TL). The specimen was identified https://doi.org/10.3391/bir.2021.10.1.18 by both morphological and molecular means. This is the species’ first record from Received: 7 June 2020 the eastern Mediterranean Sea and Greece, and only the second for the whole basin. Accepted: 16 October 2020 However, several hypotheses for potential introduction vectors are discussed, as Published: 21 December 2020 well as the potential implication to the regional lobster fishery. -

S Pin Y Lobsters
?SI S pin y Lobsters UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES WASHINGTON 25, D.C. September 1961 Fishery Leaflet 523 CONTENTS Page Introduction.............................................................................. 1 Specie 8.................................................................................... 1 Life history.............................................................................. 1 Description........................................................................ 1 Sexe s .•....................... .... ...... ..................................•......... Z Food and feeding............ ..................................................... Z Habits ....•. •... .................. .. ....•... ... ....•........... ...... ...... ... ....... 3 Molting and growth .............................................................. 3 Reproduction...................................................................... 3 The young .......................................................................... 5 Migrations............................................................................... 5 Enemie s and protection against them ............................................ 5 Capture .......... ........ ... .....•..........•........ ...... ... ..•......... ....... ...... ... 5 Utilization....... ................................ ....... ...... ...... ... ...... ..... ... ..... 6 Culture... .. .............................................................................. -

5. Index of Scientific and Vernacular Names
click for previous page 277 5. INDEX OF SCIENTIFIC AND VERNACULAR NAMES A Abricanto 60 antarcticus, Parribacus 209 Acanthacaris 26 antarcticus, Scyllarus 209 Acanthacaris caeca 26 antipodarum, Arctides 175 Acanthacaris opipara 28 aoteanus, Scyllarus 216 Acanthacaris tenuimana 28 Arabian whip lobster 164 acanthura, Nephropsis 35 ARAEOSTERNIDAE 166 acuelata, Nephropsis 36 Araeosternus 168 acuelatus, Nephropsis 36 Araeosternus wieneckii 170 Acutigebia 232 Arafura lobster 67 adriaticus, Palaemon 119 arafurensis, Metanephrops 67 adriaticus, Palinurus 119 arafurensis, Nephrops 67 aequinoctialis, Scyllarides 183 Aragosta 120 Aesop slipper lobster 189 Aragosta bianca 122 aesopius, Scyllarus 216 Aragosta mauritanica 122 affinis, Callianassa 242 Aragosta mediterranea 120 African lobster 75 Arctides 173 African spear lobster 112 Arctides antipodarum 175 africana, Gebia 233 Arctides guineensis 176 africana, Upogebia 233 Arctides regalis 177 Afrikanische Languste 100 ARCTIDINAE 173 Agassiz’s lobsterette 38 Arctus 216 agassizii, Nephropsis 37 Arctus americanus 216 Agusta 120 arctus, Arctus 218 Akamaru 212 Arctus arctus 218 Akaza 74 arctus, Astacus 218 Akaza-ebi 74 Arctus bicuspidatus 216 Aligusta 120 arctus, Cancer 217 Allpap 210 Arctus crenatus 216 alticrenatus, Ibacus 200 Arctus crenulatus 218 alticrenatus septemdentatus, Ibacus 200 Arctus delfini 216 amabilis, Scyllarus 216 Arctus depressus 216 American blunthorn lobster 125 Arctus gibberosus 217 American lobster 58 Arctus immaturus 224 americanus, Arctus 216 arctus lutea, Scyllarus 218 americanus, -

With Trachypenaeus Curvirostris (Sometimes Under the Name T
950 Shrimps and Prawns Trachypenaeus longipes (Paulson, 1875) En - Longlegged rough shrimp. Maximum body length 10.5 cm (females) and 8 cm (males). Found from nearshore waters to depths of about 220 m, usually between 40 and 60 m. Taken mainly by trawls. This species is often confused with Trachypenaeus curvirostris (sometimes under the name T. asper), and therefore its actual distribution and occurrence in the area is unclear. Probably not very common and of limited or no commercial importance. Indo-West Pacific from the Red Sea to Japan and the Philippines. distomedian distolateral projection anterior projection plate (after Motoh and Buri, 1984) posterior plate thelycum petasma (ventral view) (after Hayashi, 1992) Trachypenaeus sedili Hall, 1961 En - Singapore rough shrimp. (the FAO names previously used for this species are now used for Trachypenaeus malaiana) Maximum body length 8.8 cm (females) and 5.1 cm (males), commonly between 6 and 8 cm. Found on mud or sand bottom, from nearshore waters to depths of about 45 m.Taken by trawls and artisanal gear. Probably not a common species in the area and without commercial importance. Indo-West Pacific from India (perhaps Somalia) to the Malay Peninsula and South China Sea. anterior plate distomedian projection distolateral projection posterior plate thelycum petasma (ventral view) Penaeidae 951 Trachypenaeus villaluzi Muthu and Motoh, 1979 En - Philippines rough shrimp. Maximum body length 7.3 cm (females) and 5.3 cm (males). Caught by otter trawls at a depth of about 7 m, on mud bottom. Probably not common and without commercial importance. So far only known from the Philippines.