Disease of Aquatic Organisms 100:89
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the 23 Annual Workshop of the National Reference
Report of the 23rd Annual Workshop of the National Reference Laboratories for Fish Diseases Kgs. Lyngby, Denmark May 27th – 28th 2019 ISH staining of PRV-3 in Rainbow trout European sea bass infected with VHS heart tissue Organized by the European Union Reference Laboratory for Fish and Crustacean Diseases, National Institute of Aquatic Resources, Technical University of Denmark, Kgs. Lyngby Contents Introduction and short summary .......................................................................................... 4 Programme .......................................................................................................................... 6 SESSION I: Update on important fish diseases and their control ......................................... 10 Overview of the fish diseases situation and surveillance in Europe in 2018 ................................................................ 10 Update on fish disease situation in Norway 2018 ........................................................................................................ 14 ISA: Challenges related to epidemiology, detection, control and documentation of the ISA situation including questions related to identifying the source of new disease outbreaks .......................................................................... 16 Monitoring viral pathogens in wild brown trout in the Czech Republic ...................................................................... 18 SESSION II: Emerging diseases ......................................................................................... -

(YHV) RNA Detection by Qrt-PCR During Six-Day Storage Hongwei Ma University of Southern Mississippi
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Parasitology, Harold W. Manter Laboratory of Laboratory of Parasitology 6-2008 Stable Yellowhead Virus (YHV) RNA Detection by qRT-PCR during Six-Day Storage Hongwei Ma University of Southern Mississippi Robin M. Overstreet University of Southern Mississippi, [email protected] Jean A. Jovonovich University of Southern Mississippi, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/parasitologyfacpubs Part of the Aquaculture and Fisheries Commons, and the Parasitology Commons Ma, Hongwei; Overstreet, Robin M.; and Jovonovich, Jean A., "Stable Yellowhead Virus (YHV) RNA Detection by qRT-PCR during Six-Day Storage" (2008). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 888. http://digitalcommons.unl.edu/parasitologyfacpubs/888 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Aquaculture 278:1–4 (June 2008), pp. 10–13; doi: 10.1016/j.aquaculture.2008.03.028 Copyright © 2008 Elsevier B.V. Creative Commons Attribution Non-Commercial No Derivatives License. Submitted January 29, 2008; revised March 11, 2008; accepted March -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Viral Haemorrhagic Septicaemia Virus (VHSV): on the Search for Determinants Important for Virulence in Rainbow Trout Oncorhynchus Mykiss
Downloaded from orbit.dtu.dk on: Nov 08, 2017 Viral haemorrhagic septicaemia virus (VHSV): on the search for determinants important for virulence in rainbow trout oncorhynchus mykiss Olesen, Niels Jørgen; Skall, H. F.; Kurita, J.; Mori, K.; Ito, T. Published in: 17th International Conference on Diseases of Fish And Shellfish Publication date: 2015 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Olesen, N. J., Skall, H. F., Kurita, J., Mori, K., & Ito, T. (2015). Viral haemorrhagic septicaemia virus (VHSV): on the search for determinants important for virulence in rainbow trout oncorhynchus mykiss. In 17th International Conference on Diseases of Fish And Shellfish: Abstract book (pp. 147-147). [O-139] Las Palmas: European Association of Fish Pathologists. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. DISCLAIMER: The organizer takes no responsibility for any of the content stated in the abstracts. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -

Role of Symbiotic Bacteria on Life History Traits of Freshwater Crustacean, Daphnia Magna
Title Role of symbiotic bacteria on life history traits of freshwater crustacean, Daphnia magna Author(s) Peerakietkhajorn, Saranya Citation Issue Date Text Version ETD URL https://doi.org/10.18910/54011 DOI 10.18910/54011 rights Note Osaka University Knowledge Archive : OUKA https://ir.library.osaka-u.ac.jp/ Osaka University Doctoral Dissertation Role of symbiotic bacteria on life history traits of freshwater crustacean, Daphnia magna Saranya Peerakietkhajorn June 2015 Department of Biotechnology Graduate School of Engineering Osaka University 1 Contents Chapter 1 General introduction 5 1.1 Biology of Daphnia 6 1.2 Daphnia in bioenvironmental sciences 10 1.3 Molecular genetics of Daphnia 10 1.4 Symbiosis 11 1.5 Objective of this study 14 Chapter 2 Role of symbiotic bacteria on life history traits of D. magna and bacterial community composition 2.1 Introduction 15 2.2 Material and Methods 2.2.1 Daphnia strain and culture condition 16 2.2.2 Axenic Chlorella 17 2.2.3 Preparation of aposymbiotic juvenile Daphnia 17 2.2.4 Bacteria-free culture of aposymbiotic Daphnia 18 2.2.5 Determination of longevity of Daphnia 18 2.2.6 Re-infection by co-culture with symbiotic Daphnia 18 2.2.7 Re-infection by dipping in Daphnia extracts 18 2.2.8 DNA extraction 19 2.2.9 Quantitative polymerase chain reaction (qPCR) 19 2.2.10 Sequencing 20 2.2.11 Statistical analyse 20 2.3 Results 2 2.3.1 Generation of aposymbiotic Daphnia 20 2.3.2 Longevity of aposymbiotic Daphnia 22 2.3.3 Population dynamics of aposymbiotic Daphnia 23 2.3.4 Recovery of fecundity of aposymbiotic Daphnia by re-infection 23 2.3.5 Sequencing of symbiotic bacteria 26 2.4 Discussion 30 2.5 Summary 32 Chapter 3 Role of Limnohabitans, a dominant bacterium on D. -
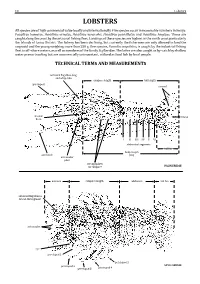
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance
Decapod crustacean grooming: Functional morphology, adaptive value, and phylogenetic significance N RAYMOND T.BAUER Center for Crustacean Research, University of Southwestern Louisiana, USA ABSTRACT Grooming behavior is well developed in many decapod crustaceans. Antennular grooming by the third maxillipedes is found throughout the Decapoda. Gill cleaning mechanisms are qaite variable: chelipede brushes, setiferous epipods, epipod-setobranch systems. However, microstructure of gill cleaning setae, which are equipped with digitate scale setules, is quite conservative. General body grooming, performed by serrate setal brushes on chelipedes and/or posterior pereiopods, is best developed in decapods at a natant grade of body morphology. Brachyuran crabs exhibit less body grooming and virtually no specialized body grooming structures. It is hypothesized that the fouling pressures for body grooming are more severe in natant than in replant decapods. Epizoic fouling, particularly microbial fouling, and sediment fouling have been shown r I m ans of amputation experiments to produce severe effects on olfactory hairs, gills, and i.icubated embryos within short lime periods. Grooming has been strongly suggested as an important factor in the coevolution of a rhizocephalan parasite and its anomuran host. The behavioral organization of grooming is poorly studied; the nature of stimuli promoting grooming is not understood. Grooming characters may contribute to an understanding of certain aspects of decapod phylogeny. The occurrence of specialized antennal grooming brushes in the Stenopodidea, Caridea, and Dendrobranchiata is probably not due to convergence; alternative hypotheses are proposed to explain the distribution of this grooming character. Gill cleaning and general body grooming characters support a thalassinidean origin of the Anomura; the hypothesis of brachyuran monophyly is supported by the conservative and unique gill-cleaning method of the group. -

A Time Series of California Spiny Lobster (Panulirus Interruptus) Phyllosoma from 1951 to 2008 Links Abundance to Warm Oceanogr
KOSLOW ET AL.: LOBSTER PHYLLOSOMA ABUNDANCE LINKED TO WARM CONDITIONS CalCOFI Rep., Vol. 53, 2012 A TIME SERIES OF CALIFORNIA SPINY LOBSTER (PANULIRUS INTERRUPTUS) PHYLLOSOMA FROM 1951 TO 2008 LINKS ABUNDANCE TO WARM OCEANOGRAPHIC CONDITIONS IN SOUTHERN CALIFORNIA J. ANTHONY KOSLOW LauRA ROGERS-BENNETT DOUGLAS J. NEILSON Scripps Institution of Oceanography California Department of Fish and Game California Department of Fish and Game University of California, S.D. Bodega Marine Laboratory 4949 Viewridge Avenue La Jolla, CA 92093-0218 UC Davis, 2099 Westside Rd. San Diego, CA 92123 ph: (858) 534-7284 Bodega Bay, CA 94923-0247 [email protected] ABSTRACT The California spiny lobster (Panulirus interruptus) population is the basis for a valuable commercial and recreational fishery off southern California, yet little is known about its population dynamics. Studies based on CalCOFI sampling in the 1950s indicated that the abun- dance of phyllosoma larvae may be sensitive to ocean- ographic conditions such as El Niño events. To further study the potential influence of environmental variabil- ity and the fishery on lobster productivity, we developed a 60-year time series of the abundance of lobster phyl- losoma from the historical CalCOFI sample collection. Phyllosoma were removed from the midsummer cruises when the early-stage larvae are most abundant in the plankton nearshore. We found that the abundance of the early-stage phyllosoma displayed considerable inter- annual variability but was significantly positively corre- Figure 1. Commercial (solid circles), recreational (open triangles), and total lated with El Niño events, mean sea-surface temperature, landings (solid line) of spiny lobster off southern California. -

FAO Fisheries Technical Paper 402/2
ISSNO0428-9345 FAO Asia Diagnostic Guide to FISHERIES TECHNICAL Aquatic Animal Diseases PAPER 402/2 NETWORK OF AQUACULTURE CENTRES IN ASIA-PACIFIC C A A N Food and Agriculture Organization of the United Nations A F O F S I I A N T P A ISSNO0428-9345 FAO Asia Diagnostic Guide to FISHERIES TECHNICAL Aquatic Animal Diseases PAPER 402/2 Edited by Melba G. Bondad-Reantaso NACA, Bangkok, Thailand (E-mail: [email protected]) Sharon E. McGladdery DFO-Canada, Moncton, New Brunswick (E-mail: [email protected]) Iain East AFFA, Canberra, Australia (E-mail: [email protected]) and Rohana P. Subasinghe NETWORK OF FAO, Rome AQUACULTURE CENTRES (E-mail: [email protected]) IN ASIA-PACIFIC C A A N Food and Agriculture Organization of the United Nations A F O F S I I A N T P A The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) or of the Network of Aquaculture Centres in Asia-Pa- cific (NACA) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its fron- tiers or boundaries. ISBN 92-5-104620-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. -

Neurolipofuscin Is a Measure of Age in the Caribbean Spiny Lobster, Panulirus Argus, in Florida
Georgia State University ScholarWorks @ Georgia State University Biology Theses Department of Biology 8-3-2006 Neurolipofuscin is a Measure of Age in the Caribbean Spiny Lobster, Panulirus argus, in Florida Kerry Elizabeth Maxwell Follow this and additional works at: https://scholarworks.gsu.edu/biology_theses Part of the Biology Commons Recommended Citation Maxwell, Kerry Elizabeth, "Neurolipofuscin is a Measure of Age in the Caribbean Spiny Lobster, Panulirus argus, in Florida." Thesis, Georgia State University, 2006. https://scholarworks.gsu.edu/biology_theses/4 This Thesis is brought to you for free and open access by the Department of Biology at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Biology Theses by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. NEUROLIPOFUSCIN IS A MEASURE OF AGE IN THE CARIBBEAN SPINY LOBSTER, PANULIRUS ARGUS, IN FLORIDA. by KERRY E. MAXWELL Under the direction of Charles D. Derby ABSTRACT Accurate age estimates for the commercially-important Caribbean spiny lobster, Panulirus argus, would greatly enhance analyses of life history and population dynamics. Previous estimates of their age based on size and growth may be inaccurate because of variable growth in the wild. An established technique for aging crustaceans – histologically-determined lipofuscin content in the nervous system – was used on lobsters reared in the laboratory for up to five years. We verified the presence of lipofuscin in eyestalk neural tissue and described its distribution in cell cluster A of the hemiellipsoid body. Neurolipofuscin content of both sexes increased linearly over the five-year age range, with seasonal oscillations. -

Balanus Trigonus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Settlement of the barnacle Balanus trigonus BRAZILIAN CRUSTACEAN SOCIETY Darwin, 1854, on Panulirus gracilis Streets, 1871, in western Mexico e-ISSN 2358-2936 www.scielo.br/nau 1 orcid.org/0000-0001-9187-6080 www.crustacea.org.br Michel E. Hendrickx Evlin Ramírez-Félix2 orcid.org/0000-0002-5136-5283 1 Unidad académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. A.P. 811, Mazatlán, Sinaloa, 82000, Mexico 2 Oficina de INAPESCA Mazatlán, Instituto Nacional de Pesca y Acuacultura. Sábalo- Cerritos s/n., Col. Estero El Yugo, Mazatlán, 82112, Sinaloa, Mexico. ZOOBANK http://zoobank.org/urn:lsid:zoobank.org:pub:74B93F4F-0E5E-4D69- A7F5-5F423DA3762E ABSTRACT A large number of specimens (2765) of the acorn barnacle Balanus trigonus Darwin, 1854, were observed on the spiny lobster Panulirus gracilis Streets, 1871, in western Mexico, including recently settled cypris (1019 individuals or 37%) and encrusted specimens (1746) of different sizes: <1.99 mm, 88%; 1.99 to 2.82 mm, 8%; >2.82 mm, 4%). Cypris settled predominantly on the carapace (67%), mostly on the gastric area (40%), on the left or right orbital areas (35%), on the head appendages, and on the pereiopods 1–3. Encrusting individuals were mostly small (84%); medium-sized specimens accounted for 11% and large for 5%. On the cephalothorax, most were observed in branchial (661) and orbital areas (240). Only 40–41 individuals were found on gastric and cardiac areas. Some individuals (246), mostly small (95%), were observed on the dorsal portion of somites.