Aimovig (Erenumab)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

New Drug Update: Not All That Glitters Is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018
9/23/2018 New Drug Update: Not All That Glitters is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018 PRESENTERS: Boise VA Medical Center Idaho State University PGY2 Ambulatory Care Residents: PGY2 Pharmacotherapy Resident: Audra Wilson, PharmD; Kat Liu, PharmD; Kailee Morton, PharmD Nitz Bankova, PharmD St. Luke’s Health System Infectious Disease Pharmacy Fellow: PGY2 Oncology Pharmacy Residents: Benjamin Pontefract, PharmD Andrew Li, PharmD; Amanda Wright, PharmD; Bryce Benson, PharmD PGY2 Mental Health Pharmacy Resident: Samantha Patton, PharmD Disclosures No conflicts of interest to disclose Learning Objectives • Describe clinical scenarios where newly approved medications are beneficial. • Compare newly approved medications with current standards of care. • Identify investigational new drugs and their potential clinical applications. 1 9/23/2018 Overview of Topics • Infectious Disease • Ambulatory Care • Diabetes Mellitus • Women’s Health • Anticoagulation • Pain Management • Oncology • Acute Myeloid Leukemia (AML) Infectious Disease: Plazomicin Delafloxacin Benjamin Pontefract, PharmD Plazomicin (Zemdri®):Overview • Systemic antibiotic in the aminoglycoside family • MoA: disrupts the 30s ribosome to prevent protein synthesis • Concentration dependent bactericidal activity • Approved to treat UTIs caused by E. coli, K. pneumoniae, Proteus spp, and Enterobacter cloaecae 2 9/23/2018 Aminoglycoside Shortcomings Toxicities Resistance • Nephrotoxicity • Decreased cell permeability • Ototoxicity • Altered ribosome binding sites • Therapeutic drug monitoring • AMG‐modifying enzymes (AME) Plazomicin: Efficacy • Study 009: • RCT comparing plazomicin IV to meropenem IV for cUTI caused by enterobactereceae • Plazomicin group was non‐inferior to meropenem group • Study 007: • RCT comparing plazomicin IV to polymyxin E IV for BSI cause by carbapenem‐resistant Enterobacteriaceae (CRE) • Plazomicin was associated with less mortality compared to polymyxin E Connolly L, Achaogen, Inc. -

NCT04179474 Study ID: 3110‐108‐002 Title: A
NCT04179474 Study ID: 3110‐108‐002 Title: A Phase 1b, Two‐Part, Open‐Label, Fixed‐Sequence, Safety, Tolerability and Drug‐Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Date: 14Aug2019 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Title Page Protocol Title: A Phase 1b, Two-Part, Open-Label, Fixed-Sequence, Safety, Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Number: 3110-108-002 Product: Ubrogepant (AGN-241688, MK-1602) Brief Protocol Title: Drug-Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab Study Phase: 1b Sponsor Name and Legal Registered Address: Allergan Pharmaceuticals International Limited, Clonshaugh Industrial Estate, Coolock, Dublin 17, Ireland US Agent: Allergan Sales, LLC, 2525 Dupont Dr, Irvine, California 92612, USA Regulatory Agency Identifying Number(s): IND #113924 SAE Reporting Fax Number/Email: Business Unit Email Phone Fax Allergan Approval Date: 14 August 2019 1 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Sponsor Signatories: Date Date The signatures of the sponsor signatories are collected on the protocol approval page. 2 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Table of Contents Title Page .........................................................................................................................................1 -
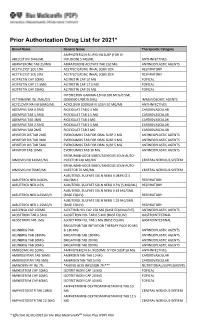
Prior Authorization Drug List for 2021*
Prior Authorization Drug List for 2021* Brand Name Generic Name Therapeutic Category AMPHOTERICIN B LIPID INJ SUSP (FOR IV ABELCET INJ 5MG/ML INFUSION) 5 MG/ML ANTI-INFECTIVES ABIRATERONE TAB 250MG ABIRATERONE ACETATE TAB 250 MG ANTINEOPLASTIC AGENTS ACETYLCYST SOL 10% ACETYLCYSTEINE INHAL SOLN 10% RESPIRATORY ACETYLCYST SOL 20% ACETYLCYSTEINE INHAL SOLN 20% RESPIRATORY ACITRETIN CAP 10MG ACITRETIN CAP 10 MG TOPICAL ACITRETIN CAP 17.5MG ACITRETIN CAP 17.5 MG TOPICAL ACITRETIN CAP 25MG ACITRETIN CAP 25 MG TOPICAL INTERFERON GAMMA-1B INJ 100 MCG/0.5ML ACTIMMUNE INJ 2MU/0.5 (2000000 UNIT/0.5ML) IMMUNOLOGIC AGENTS ACYCLOVIR NA INJ 50MG/ML ACYCLOVIR SODIUM IV SOLN 50 MG/ML ANTI-INFECTIVES ADEMPAS TAB 0.5MG RIOCIGUAT TAB 0.5 MG CARDIOVASCULAR ADEMPAS TAB 1.5MG RIOCIGUAT TAB 1.5 MG CARDIOVASCULAR ADEMPAS TAB 1MG RIOCIGUAT TAB 1 MG CARDIOVASCULAR ADEMPAS TAB 2.5MG RIOCIGUAT TAB 2.5 MG CARDIOVASCULAR ADEMPAS TAB 2MG RIOCIGUAT TAB 2 MG CARDIOVASCULAR AFINITOR DIS TAB 2MG EVEROLIMUS TAB FOR ORAL SUSP 2 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 3MG EVEROLIMUS TAB FOR ORAL SUSP 3 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 5MG EVEROLIMUS TAB FOR ORAL SUSP 5 MG ANTINEOPLASTIC AGENTS AFINITOR TAB 10MG EVEROLIMUS TAB 10 MG ANTINEOPLASTIC AGENTS ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 140MG/ML INJECTOR 140 MG/ML CENTRAL NERVOUS SYSTEM ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 70MG/ML INJECTOR 70 MG/ML CENTRAL NERVOUS SYSTEM ALBUTEROL SULFATE SOLN NEBU 0.083% (2.5 ALBUTEROL NEB 0.083% MG/3ML) RESPIRATORY ALBUTEROL NEB 0.5% ALBUTEROL SULFATE -

761119Orig1s000 SUMMARY REVIEW
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761119Orig1s000 SUMMARY REVIEW Summary Review Summary Review Date February 21, 2020 Heather Fitter, MD From Nick Kozauer, MD Billy Dunn, MD Subject Summary Review BLA # 761119 Applicant Lundbeck Seattle BioPharmaceuticals, Inc. Date of Submission December 26, 2018 PDUFA Goal Date February 21, 2020 Proprietary Name Vyepti Established or Proper Names Eptinezumab Solution for intravenous (IV) injection (100 Dosage Form mg/mL) Applicant Proposed Prevention of migraine in adults Indication/Population 100 mg IV infusion every 3 months; 300 mg IV Applicant Proposed Dosing Regimen(s) infusion every 3 months Recommendation on Regulatory Action Approval Recommended Indication/Population Preventive treatment of migraine in adults 100 mg IV infusion every 3 months; 300 mg IV Recommended Dosing Regimen(s) infusion every 3 months 1 Reference ID: 4564908 Summary Review 1. Benefit-Risk Assessment Benefit-Risk Assessment Framework Benefit-Risk Integrated Assessment Eptinezumab is a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand, and prevents CGRP binding to its receptor. The applicant provided information supporting safety and efficacy in patients with both chronic migraine (i.e., at least 15 headache days/month, with features of migraine headache on at least 8 days/month) and episodic migraine (i.e., up to 14 migraine headache days/month). There are several FDA-approved drugs for the preventive treatment of migraine. Three drugs in the anti-CGRP class were approved recently for this indication: erenumab (May 2018), fremanezumab (September 2018), and galcanezumab (September 2018). These three drugs are given by subcutaneous (SC) administration either monthly or quarterly, while eptinezumab is given intravenously (IV) quarterly. -

Aimovig, INN-Erenumab
Part VI: Summary of the risk management plan Summary of risk management plan for Aimovig (Erenumab) This is a summary of the risk management plan (RMP) for Aimovig. The RMP details important risks of Aimovig, how these risks can be minimized, and how more information will be obtained about Aimovig’s risks and uncertainties (missing information). Aimovig’s summary of product characteristics (SmPC) and its package leaflet give essential information to healthcare professionals and patients on how Aimovig should be used. This summary of the RMP for Aimovig should be read in the context of all this information including the assessment report of the evaluation and its plain-language summary, all which is part of the European Public Assessment Report (EPAR). Important new concerns or changes to the current ones will be included in updates of Aimovig’s RMP. I. The medicine and what it is used for Aimovig is authorized for the prophylaxis of migraine in adults who have at least 4 migraine days per month (see SmPC for the full indication). It contains erenumab (a human IgG2 monoclonal antibody) as the active substance and it is given by sc injections. Further information about the evaluation of Aimovig’s benefits can be found in Aimovig’s EPAR, including in its plain-language summary, available on the EMA website, under the medicine’s webpage: http://www.ema.europa.eu/ema/index.jsp?curl=/pages/medicines/human/medicines/004447/hum an_med_002275.jsp. II. Risks associated with the medicine and activities to minimize or further characterize the risks Important risks of Aimovig, together with measures to minimize such risks and the proposed studies for learning more about Aimovig's risks, are outlined below in Table 2. -

761077Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761077Orig1s000 OTHER REVIEW(S) Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research | Office of Surveillance and Epidemiology (OSE) Epidemiology: ARIA Sufficiency Templates Version: 2018-01-24 Date: May 15, 2018 Reviewer(s): Hongliu Ding, MD, PhD, MPH Division of Epidemiology I Team Leader: Kira Leishear, PhD, MS Division of Epidemiology I Division Deputy Director: Sukhminder K. Sandhu, PhD, MS, MPH Division of Epidemiology I Subject: ARIA Sufficiency Memo for Pregnancy Safety Concerns Drug Name(s): Amovig (erenumab) Application Type/Number: BLA 761077 Applicant/sponsor: Amgen Inc. OSE RCM #: 2018-869 Page 1 of 5 Reference ID: 4263424 Expedited ARIA Sufficiency Template for Pregnancy Safety Concerns 1. BACKGROUND INFORMATION 1.1. Medical Product Amovig (erenumab) is a human immunoglobulin G2 (IgG2) monoclonal antibody against the calcitonin gene-related peptide (CGRP) receptor. CGRP is a neuropeptide that modulates nociceptive signaling and a vasodilator associated with migraine pathophysiology.1-3 Plasma CGRP levels have been shown to increase significantly during migraine and return to normal when headache is relieved.4, 5 Erenumab competes with the binding of CGRP and inhibits its function at the CGRP receptor, and thus, the proposed indication is for prophylaxis of episodic and chronic migraine in adults. 1.2. Describe the Safety Concern Amovig (erenumab) exposure to women affected by migraine that are pregnant or of childbearing potential is possible. The data from a non-clinical study provided by the sponsor in which female monkeys were administered erenumab (0 or 50 mg/kg) twice weekly by subcutaneous showed that injection throughout pregnancy (gestation day 20-22 to parturition) did not observe adverse effects on offspring. -

CDER List of Licensed Biological Products With
Center for Drug Evaluation and Research List of Licensed Biological Products with (1) Reference Product Exclusivity and (2) Biosimilarity or Interchangeability Evaluations to Date DATE OF FIRST REFERENCE PRODUCT DATE OF LICENSURE LICENSURE EXCLUSIVITY EXPIRY DATE INTERCHANGEABLE (I)/ BLA STN PRODUCT (PROPER) NAME PROPRIETARY NAME (mo/day/yr) (mo/day/yr) (mo/day/yr) BIOSIMILAR (B) WITHDRAWN 125118 abatacept Orencia 12/23/05 NA NA 103575 abciximab ReoPro 12/22/94 NA NA Yes 125274 abobotulinumtoxinA Dysport 04/29/09 125057 adalimumab Humira 12/31/02 NA NA 761071 adalimumab-adaz Hyrimoz 10/30/18 B 761058 adalimumab-adbm Cyltezo 08/25/17 B 761118 adalimumab-afzb Abrilada 11/15/19 B 761024 adalimumab-atto Amjevita 09/23/16 B 761059 adalimumab-bwwd Hadlima 07/23/19 B 125427 ado-trastuzumab emtansine Kadcyla 02/22/13 125387 aflibercept Eylea 11/18/11 103979 agalsidase beta Fabrazyme 04/24/03 NA NA 125431 albiglutide Tanzeum 04/15/14 017835 albumin chromated CR-51 serum Chromalbin 02/23/76 103293 aldesleukin Proleukin 05/05/92 NA NA 103948 alemtuzumab Campath, Lemtrada 05/07/01 NA NA 125141 alglucosidase alfa Myozyme 04/28/06 NA NA 125291 alglucosidase alfa Lumizyme 05/24/10 125559 alirocumab Praluent 07/24/15 103172 alteplase, cathflo activase Activase 11/13/87 NA NA 103950 anakinra Kineret 11/14/01 NA NA 020304 aprotinin Trasylol 12/29/93 125513 asfotase alfa Strensiq 10/23/15 101063 asparaginase Elspar 01/10/78 NA NA 125359 asparaginase erwinia chrysanthemi Erwinaze 11/18/11 761034 atezolizumab Tecentriq 05/18/16 761049 avelumab Bavencio 03/23/17 -

P&T Summary 2Q2019
BLUE SHIELD OF CALIFORNIA SECOND QUARTER 2019 FORMULARY AND MEDICATION POLICY UPDATES EFFECTIVE AUGUST 1, 2019 for Large Group, Small Group, and Individual & Family Plans The Blue Shield of California (BSC) Pharmacy and Therapeutics (P&T) Committee, consisting of network physicians and pharmacists, regularly evaluates drugs for formulary inclusion and medication policy coverage criteria. The second quarter 2019 P&T Committee decisions on formulary changes and medication policy changes, which apply to Commercial members with an outpatient drug benefit, are summarized below: PHARMACY BENEFIT FORMULARY UPDATE: Please refer to the appropriate drug formulary posted on our website for the following information: • Quantity limits, if applicable, for specific drugs • Formulary status of newly available strengths of existing drugs. Note: The formulary status of newly available strengths of existing drugs will have the same formulary status as the existing strengths unless otherwise noted. • Non-formulary and non-preferred generic drugs that do not require prior authorization or step therapy • Brand-name medications that are now non-formulary or non-preferred because these medications have newly available generic equivalents covered on the formulary Formularies are available at blueshieldca.com/pharmacy. Select the appropriate drug formulary – “Standard Drug Formulary” or “Plus Drug Formulary”. Summary of changes to the Medicare formularies are available at blueshieldca.com/pharmacy. Select “Medicare Drug Formulary”, then select the appropriate -

Umpqua Health Alliance List of Medication Coverage Changes
Umpqua Health Alliance List of Medication Coverage Changes (Updated 4/1/2021) INTRODUCTION The Umpqua Health Alliance (UHA) Pharmacy and Therapeutics (P&T) Committee composed of pharmacists and physicians determine which drugs should be covered, the coverage restrictions, and the PA guidelines. The goal of the P&T Committee is to create a formulary (list of covered drugs) with medications that are safe and effective and that offer the best value. Our formulary and prior authorization guidelines are usually updated quarterly. This document contains all updates made to the UHA formulary and PA guidelines since January 1, 2020. The current versions of our formulary and PA guidelines are available on the UHA Pharmacy Services webpage: https://www.umpquahealth.com/pharmacy‐services/. LEGEND The following are restriction and coverage abbreviations found in this document: ABBREVIATIONS DEFINITION EXPLANATION PA Prior Authorization Required Prior authorization (e.g. prior approval) is required before filling a prescription for this drug. Without prior authorization, we may not cover this drug. The provider must submit a request for prior authorization with the appropriate documentation (including recent chart notes) before the drug is covered. A specific PA guideline policy number beginning with “RX” may be referenced in the “Description” column. Visit the UHA Pharmacy Services webpage for our PA guidelines: https://www.umpquahealth.com/pharmacy‐services/. ST Step Therapy Restriction We require trial and failure of one or more lower‐cost or preferred drug(s) (“Step 1 drug”) before using the more expensive or non‐preferred drug (“Step 2 drug”). If it is medically necessary for a member to use a Step 2 drug first, the prescriber will need to submit a request for prior authorization. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

CGRP and Headache: a Brief Review
Neurological Sciences https://doi.org/10.1007/s10072-019-03769-8 REVIEW ARTICLE CGRP and headache: a brief review Stewart J. Tepper1,2 # Fondazione Società Italiana di Neurologia 2019 Abstract The advent of anti-CGRP medications is an example of translational research made real. Pioneering research by Drs. Lars Edvinsson and Peter Goadsby has yielded the monoclonal antibody therapeutics and will likely also result in the gepants. The availability of MABs represents a watershed moment in the treatment of migraine. These medications have specificity, as they were designed for primary migraine prevention. They work across a group of wide therapeutic targets, episodic migraine, chronic migraine, medication-overuse headache, and episodic cluster headache. They separate from placebo within 1 week, and often show clinical effects within a month or less. They have tolerability similar to placebo. There has been no significant or worrisome safety signal thus far in their use. They manifest unprecedented responder rates at ≥ 75% and even 100%. They lower all acute medication use and can convert patients from chronic migraine to episodic migraine and from acute medication overuse to non- overuse. They work in patients who have already had lack of success with at least 2–4 previous preventive medications. Pent-up demand for designer, well-tolerated, and effective migraine preventive medication in the USA has resulted in more than 100,000 individual patients prescribed erenumab from May to December of 2018, and the numbers continue to increase. The preventive treatment of migraine in the USA has shifted dramatically, and is likely to do so in the rest of the world as well.