2020.02.23.20026856V2.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ningbo Facts
World Bank Public Disclosure Authorized Climate Resilient Ningbo Project Local Resilience Action Plan 213730-00 Final | June 2011 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan Contents Page 1 Executive Summary 4 2 Introduction 10 3 Urban Resilience Methodology 13 3.1 Overview 13 3.2 Approach 14 3.3 Hazard Assessment 14 3.4 City Vulnerability Assessment 15 3.5 Spatial Assessment 17 3.6 Stakeholder Engagement 17 3.7 Local Resilience Action Plan 18 4 Ningbo Hazard Assessment 19 4.1 Hazard Map 19 4.2 Temperature 21 4.3 Precipitation 27 4.4 Droughts 31 4.5 Heat Waves 32 4.6 Tropical Cyclones 33 4.7 Floods 35 4.8 Sea Level Rise 37 4.9 Ningbo Hazard Analysis Summary 42 5 Ningbo Vulnerability Assessment 45 5.1 People 45 5.2 Infrastructure 55 5.3 Economy 69 5.4 Environment 75 5.5 Government 80 6 Gap Analysis 87 6.1 Overview 87 6.2 Natural Disaster Inventory 87 6.3 Policy and Program Inventory 89 6.4 Summary 96 7 Recommendations 97 7.1 Overview 97 7.2 People 103 7.3 Infrastructure 106 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan 7.4 Economy 112 7.5 Environment 115 7.6 Government 118 7.7 Prioritized Recommendations 122 8 Conclusions 126 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan List of Tables Table -

18 F, China Life Tower, No. 777 Lingqiao Road, Haishu District, Ningbo
18 F, China Life Tower, No. 777 Lingqiao Road, Haishu District, Ningbo View this office online at: https://www.newofficeasia.com/details/serviced-offices-18-f-china-life-tower-n o-777-lingqiao-road-haishu-district China Life Tower is an iconic and highly visible building which dominates the skyline and boasts LEED gold rating and 360 degree unobstructed views of the surrounding rivers. The serviced offices reside on the 18th floor and offer a quiet yet professional working environment which is equipped with the latest technology to help your business thrive. Stylish kitchen/diner facilities are available alongside executive meeting rooms, all of which can be accessed day or night and are protected by 24-hour security personnel for your safety. Transport links Nearest airport: Key features 24 hour access 24-hour security Car parking spaces Comfortable lounge Disabled facilities (DDA/ADA compliant) High-speed internet Lift Meeting rooms Suspended Ceilings Voicemail Location Located adjacent to the Fenghua River, your company can enjoy stunning riverside views and easy access to a plethora of local amenities. There is a popular shopping district nearby which is home to a diverse range of retailers, restaurants, banks and hotels. The street is well-served by bus routes and the metro line resides within easy walking distance while Ningbo Lishe International Airport can be reached within a 25 minute drive. Points of interest within 1000 metres China Everbright Bank (bank) - 150m from business centre Citic Hotel (hotel) - 366m from business centre Shangri-la -

A Survey of Marine Coastal Litters Around Zhoushan Island, China and Their Impacts
Journal of Marine Science and Engineering Article A Survey of Marine Coastal Litters around Zhoushan Island, China and Their Impacts Xuehua Ma 1, Yi Zhou 1, Luyi Yang 1 and Jianfeng Tong 1,2,3,* 1 College of Marine Science, Shanghai Ocean University, Shanghai 201306, China; [email protected] (X.M.); [email protected] (Y.Z.); [email protected] (L.Y.) 2 National Engineering Research Center for Oceanic Fisheries, Shanghai 201306, China 3 Experimental Teaching Demonstration Center for Marine Science and Technology, Shanghai Ocean University, Shanghai 201306, China * Correspondence: [email protected] Abstract: Rapid development of the economy increased marine litter around Zhoushan Island. Social- ecological scenario studies can help to develop strategies to adapt to such change. To investigate the present situation of marine litter pollution, a stratified random sampling (StRS) method was applied to survey the distribution of marine coastal litters around Zhoushan Island. A univariate analysis of variance was conducted to access the amount of litter in different landforms that include mudflats, artificial and rocky beaches. In addition, two questionnaires were designed for local fishermen and tourists to provide social scenarios. The results showed that the distribution of litter in different landforms was significantly different, while the distribution of litter in different sampling points had no significant difference. The StRS survey showed to be a valuable method for giving a relative overview of beach litter around Zhoushan Island with less effort in a future survey. The questionnaire feedbacks helped to understand the source of marine litter and showed the impact on the local environment and economy. -
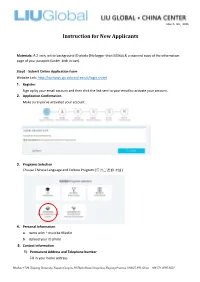
ZJU Instructions for New Applicants
March 5th, 2020 Instruction for New Applicants Materials: A 2-inch, white background ID photo (No bigger than 500kb) & a scanned copy of the information page of your passport (under 1mb in size). Step1 - Submit Online Application Form Website Link: http://isinfosys.zju.edu.cn/recruit/login.shtml 1. Register: Sign up by your email account and then click the link sent to your email to activate your account. 2. Application Confirmation Make sure you’ve activated your account. 3. Programs Selection Choose Chinese Language and Culture Program (汉语言进修项目). 4. Personal Information a. Items with * must be filled in b. Upload your ID photo 5. Contact Information 1) Permanent Address and Telephone Number Fill in your home address Mailbox 1709, Zhejiang University, Yuquan Campus, 38 Zheda Road, Hangzhou, Zhejiang Province 310027, P.R. China +86 571 8795 2051 March 5th, 2020 2) Address to Receive Admission Documents & Telephone Number a. If you are in the States, please fill in your mailing address. (please fill in the English address, otherwise it will affect the accurate postal delivery.) b. If you are out of the States, please fill in the address of China Center. (Copy the information below) Country – China City - HangZhou Postal Code – 310027 Address – Room 303, Building 11, 38 Zheda Road, Zhejiang University Yuquan Campus, Hangzhou, Zhejiang Province, P.R. China 3) Current Contacts (Copy the information below) Mailbox 1709, Zhejiang University, Yuquan Campus, 38 Zheda Road, Hangzhou, Zhejiang Province 310027, P.R. China +86 571 8795 2051 March 5th, 2020 Emergency Contact Person – Danyang (Ann) Zheng Emergency Contact Phone Number -13777886407 Emergency Contact Address - Room 303, Building 11, 38 Zheda Road, Zhejiang U niversity Yuquan Campus, Hangzhou, Zhejiang Province, P.R. -

Critical Language Scholarship Program
CRITICAL LANGUAGE SCHOLARSHIP PROGRAM Hangzhou CHINA HANDBOOK FOR PARTICIPANTS SUMMER 2014 The CLS Program is a program of the U.S. Department of State’s Bureau of Educational and Cultural Affairs. The CLS Program in China is administered by the Department of East Asian Languages and Literatures, at The Ohio State University. The Ohio State University 398 Hagerty Hall 1775 College Rd. Columbus, OH 43210-1298 Photo courtesy of Prof. Kirk Denton, Department of East Asian Languages The Ohio State University This handbook was compiled and edited by staff of the Critical Language Scholarship East Asian Languages Program at the Ohio State University and adapted from CLS handbooks from American Councils for International Education Contents Contents ......................................................................................................................................................... i Section I: Introduction .................................................................................................................................. 6 Welcome ................................................................................................................................................... 6 Fast Facts ................................................................................................................................................... 6 CLS Program ........................................................................................................................................ 6 Program Staff ....................................................................................................................................... -

Request for Recruitment Program of Foreign Experts Zheijang Final
EDUCATION & RESEARCH Request for Recruitment Program of Foreign Experts 1 Client : Zhejiang Normal University Industry : Higher Education Region : Jinhua, Zhejiang Province Zhejiang Normal University(ZNU) is one of the key universities of Zhejiang Province. ZNU emphasizes in teacher education with multiple branches of learning. The University consists of 19 colleges offering 61 undergraduate programs. It has an enrolment over Client Information (main 25,700 undergraduates, 5,000 postgraduates, and 15,000 adult students in various adult areas of activity, etc. ): education programs. The total staff is about 2,700. In 2009, ZNU became a doctoral degree project construction unit. Now there are 23 senior subject master degree programs and 11 professional degree Master programs. There are complete infrastructure, rich library resources, and advanced equipment in ZNU. 1. Leverage the key discipline platform of pure and applied mathematics to strengthen international and mainland academic exchange. Invite internationally well-known scholars to visit, give seminars or short courses to introduce research results and directions in Nature of Activity frontiers of mathematics. Elevate discipline’s international recognition and influence. Requirement for 2. Carefully select research topics. Choose topics with theoretical depth or those with Experts (main task, goal prospect of wide application and in the mainstream of mathematics with research content keeping with international frontiers. Initiate and develop new research direction. to be attained, etc. ): 3. Train high quality researchers. Strengthen the training of graduate students and young faculty members; improve their creativity and implementation skills. Thru short courses to systematically introduce the current state of research, research topics and research methods. Broaden the horizon and area of our research team. -

Vertical Metal F'ile Cabinets
Barcode:3827074-03 C-570-111 INV - Investigation - CHINESE EXPORTERS AND PRODUCERS OF VERTICAL METAL F'ILE CABINETS Best Beaufy Furniture Co., Ltd. Feel Life Co., Ltd. Lianping Industry Zone, Dalingshan Town Room 202, Deweisen Building Dongguan, Guangdon g, 523809 Nanshan District, Shenzhen Tel: +86 769-85623639 1sf ¡ +86 7 55-66867080-8096 Fax: +86 769-85628609 Fax: +86 755-86146992 Email: N/A Email : [email protected] Website: Website : www. feellife. com www. d gbestbeauty. company. weiku. com/ Fujian lvyer Industrial Co., Ltd. Chung \ilah Steel Furniture Factory Co., Yangxia Village, Guhuai Town Lrd. Changle, Fujian, 350207 Block A,7lF Chinaweal Centre Tel: +86 59128881270 414-424 Jaffe Road, Wanchai Fax: +86 59128881315 Hong Kong Email : nancy @flivyer. com Tel: +85 228930378 Website : www. ivyer.net. cnl Fax: +85 228387626 Email: N/A Fuzhou Nu Deco Crafts Co., Ltd. Website : http ://chungwah. com.hk/ 1306 Xinxing Building No. 41, Bayiqi Mid. Road Concept Furniture (Anhui) Co.' Ltd. Fuzhou, Fujian, 350000 Guangde Economic and Technical Tel: +86 591-87814422 Developm ent Zone, Guangde County P¿¡; +86 591-87814424 Anhui, Xuancheng, 242200 Email: [email protected] Tel: 865-636-0131 Website : http :/inu-deco.cnl Fax: 865-636-9882 Email: N/A Fuzhou Yibang Furniture Co., Ltd. Website: N/A No. 85-86 Building Changle Airport Industrial Zone Dong Guan Shing Fai X'urniture Hunan Town, Changle 2nd Industrial Area Fujian, Fuzhou, 350212 Shang Dong Administrative Dist. fsf; +86 591-28637056 Qishi, Dongguan, Guangdong, 523000 Fax: +86 591-22816378 Tel: +86 867592751816 Email: N/A Fax: N/A V/ebsite : htþs ://fi yb. -

Shop Direct Factory List Dec 18
Factory Factory Address Country Sector FTE No. workers % Male % Female ESSENTIAL CLOTHING LTD Akulichala, Sakashhor, Maddha Para, Kaliakor, Gazipur, Bangladesh BANGLADESH Garments 669 55% 45% NANTONG AIKE GARMENTS COMPANY LTD Group 14, Huanchi Village, Jiangan Town, Rugao City, Jaingsu Province, China CHINA Garments 159 22% 78% DEEKAY KNITWEARS LTD SF No. 229, Karaipudhur, Arulpuram, Palladam Road, Tirupur, 641605, Tamil Nadu, India INDIA Garments 129 57% 43% HD4U No. 8, Yijiang Road, Lianhang Economic Development Zone, Haining CHINA Home Textiles 98 45% 55% AIRSPRUNG BEDS LTD Canal Road, Canal Road Industrial Estate, Trowbridge, Wiltshire, BA14 8RQ, United Kingdom UK Furniture 398 83% 17% ASIAN LEATHERS LIMITED Asian House, E. M. Bypass, Kasba, Kolkata, 700017, India INDIA Accessories 978 77% 23% AMAN KNITTINGS LIMITED Nazimnagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 1708 60% 30% V K FASHION LTD formerly STYLEWISE LTD Unit 5, 99 Bridge Road, Leicester, LE5 3LD, United Kingdom UK Garments 51 43% 57% AMAN GRAPHIC & DESIGN LTD. Najim Nagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 3260 40% 60% WENZHOU SUNRISE INDUSTRIAL CO., LTD. Floor 2, 1 Building Qiangqiang Group, Shanghui Industrial Zone, Louqiao Street, Ouhai, Wenzhou, Zhejiang Province, China CHINA Accessories 716 58% 42% AMAZING EXPORTS CORPORATION - UNIT I Sf No. 105, Valayankadu, P. Vadugapal Ayam Post, Dharapuram Road, Palladam, 541664, India INDIA Garments 490 53% 47% ANDRA JEWELS LTD 7 Clive Avenue, Hastings, East Sussex, TN35 5LD, United Kingdom UK Accessories 68 CAVENDISH UPHOLSTERY LIMITED Mayfield Mill, Briercliffe Road, Chorley Lancashire PR6 0DA, United Kingdom UK Furniture 33 66% 34% FUZHOU BEST ART & CRAFTS CO., LTD No. 3 Building, Lifu Plastic, Nanshanyang Industrial Zone, Baisha Town, Minhou, Fuzhou, China CHINA Homewares 44 41% 59% HUAHONG HOLDING GROUP No. -

2019 Interim Results Presentation
Agenda 01 Result Highlights 02 Review of Operations 03 Outlook & Growth Strategies 04 Appendices 2 Result Highlights Result Highlights 1H2019 1H2018 % Change Results (unaudited) Rmb ’000 (Restated) Revenue 5,722,101 5,390,447 6.2% – Including: Interest income 787,455 704,445 11.8% Operating costs (2,925,250) (2,590,416) 12.9% Gross profit 2,796,851 2,800,031 -0.1% Securities investment gains 658,810 142,049 363.8% Other income and gains and losses 103,789 277,729 -62.6% Administrative expenses (46,732) (49,353) -5.3% Other expenses (39,656) (39,514) 0.4% (Recognition) reversal of impairment losses, net (2,688) 20,595 N/A Share of profit of associates 327,447 136,133 140.5% Share of profit of a joint venture 12,189 11,652 4.6% Finance costs (767,975) (652,747) 17.7% Profit before tax 3,042,035 2,646,575 14.9% Income tax expense (692,971) (573,285) 20.9% Profit for the Period 2,349,064 2,073,290 13.3% – Attributable to owners of the Company 1,977,610 1,835,053 7.8% – Attributable to non-controlling interests 371,454 238,237 55.9% EPS (basic) (RMB cents) 45.53 42.25 7.8% EPS (diluted) (RMB cents) 44.47 37.97 17.1% 4 Result Highlights – Revenue Revenue Revenue Breakdown Rmb million 14,000 Toll Road Business 11,874 12,000 10,979 11,081 11,192 67.8% 10,000 8,000 5,824 5,722 6,000 5,372 5,336 5,390 4,000 Other Business Securities Business 2,000 3.5% 2015 2016 2017 2018 2019 28.7% (Restated) (Restated) (Restated) (Restated) Interim Annual Overall Revenue for the Group increased 6.2% y-o-y to Rmb5,722 million 5 Result Highlights – Net Profit Net -

Nbo Apr Schedule 3月24, 2021
TP Remarks: 2021 Apr Vessel Schdule Export 1. Attention To China Customs Advance Manifest (CCAM) Regulation : Please submit CCAM to China Customs 24 hours prior to cargo loading on vessels sailing to/from China mainland ports. Last Updated at: 23-Mar-2021 2. Schedules are subject to change without prior notice. Ocean Network Express (China) Ltd. Room 1103-1106, 11F,China life Building,NO.777,Lingqiao Road, Haishu District, Ningbo City,Zhejiang Province 北美航线 PS6(Direct Service) Ningbo 二期(NBCT) ETA USLAX ETA OAKLAND WK VSL NAME VOY CY Cutoff AMS Cutoff VGM Cutoff ETD CY Open 08:00 AM 12:00 AM 15天 21天 ETB - 24 Hours(ETB 14 CZECH 101E Per terminal SUN SAT 时间以港区网站为准) Wed. 7-Apr 22-Apr 28-Apr ETB - 24 Hours(ETB 15 KUALA LUMPUR EXPRESS 089E Per terminal SUN SAT 时间以港区网站为准) Wed. 14-Apr 29-Apr 5-May ETB - 24 Hours(ETB 16 MOL CELEBRATION 082E Per terminal SUN SAT 时间以港区网站为准) Wed. 21-Apr 6-May 12-May ETB - 24 Hours(ETB 17 SEASPAN ADONIS 062E Per terminal SUN SAT 时间以港区网站为准) Wed. 28-Apr 13-May 19-May 船代 : 兴港 (Ningbo Xinggang) 北美航线 PS5(Direct Service) Ningbo 三期(NINGBO ZHOUSHAN PORT CO.LTD BEILUN SECOND CONTAINER TERMINAL BRANCH) ETA USLAX WK VSL NAME VOY CY Cutoff AMS Cutoff VGM Cutoff ETD CY Open 16:00 PM 17:00 PM 13天 ETB - 24 Hours(ETB 14 HYUNDAI SPLENDOR 082E Per terminal FRI THU 时间以港区网站为准) Mon. 5-Apr 18-Apr ETB - 24 Hours(ETB 15 VMS Per terminal FRI THU 时间以港区网站为准) Mon. 12-Apr 25-Apr ETB - 24 Hours(ETB 16 MOL PRESTIGE 066E Per terminal FRI THU 时间以港区网站为准) Mon. -

Barcode:3844251-01 A-570-112 INV - Investigation
Barcode:3844251-01 A-570-112 INV - Investigation - PRODUCERS AND EXPORTERS FROM THE PRC Producer/Exporter Name Mailing Address A-Jax International Co., Ltd. 43th Fei Yue Road, Zhongshan City, Guandong Province, China Anhui Amigo Imp.&Exp. Co., Ltd. Private Economic Zone, Chaohu, 238000, Anhui, China Anhui Sunshine Stationery Co., Ltd. 17th Floor, Anhui International Business Center, 162, Jinzhai Road, Hefei, Anhui, China Anping Ying Hang Yuan Metal Wire Mesh Co., Ltd. No. 268 of Xutuan Industry District of Anping County, Hebei Province, 053600, China APEX MFG. CO., LTD. 68, Kuang-Chen Road, Tali District, Taichung City, 41278, Taiwan Beijing Kang Jie Kong 9-2 Nanfaxin Sector, Shunping Rd, Shunyi District, Beijing, 101316, China Changzhou Kya Fasteners Co., Ltd. Room 606, 3rd Building, Rongsheng Manhattan Piaza, Hengshan Road, Xinbei District, Changzhou City, Jiangsu, China Changzhou Kya Trading Co., Ltd. Room 606, 3rd Building, Rongsheng Manhattan Piaza, Hengshan Road, Xinbei District, Changzhou City, Jiangsu, China China Staple #8 Shu Hai Dao, New District, Economic Development Zone, Jinghai, Tianjin Chongqing Lishun Fujie Trading Co., Ltd. 2-63, G Zone, Perpetual Motor Market, No. 96, Torch Avenue, Erlang Technology New City, Jiulongpo District, Chongqing, China Chongqing Liyufujie Trading Co., Ltd. No. 2-63, Electrical Market, Torch Road, Jiulongpo District, Chongqing 400000, China Dongyang Nail Manufacturer Co.,Ltd. Floor-2, Jiaotong Building, Ruian, Wenzhou, Zhejiang, China Fastco (Shanghai) Trading Co., Ltd. Tong Da Chuang Ye, Tian -

'Yongjin', 'Xiaguang' and 'Yuhuang': Three Ornamental Cultivars Of
HORTSCIENCE 50(5):762–764. 2015. and Forestry University. These cultivars exhibit yellow or red leaves that stay on the branch for a longer period. These characteristics provide ‘Yongjin’, ‘Xiaguang’ and ‘Yuhuang’: higher ornamental value to the cultivars com- Three Ornamental Cultivars of pared with common camphor trees. Cinnamomum camphora Origin In Nov. 1998, we conducted a survey on Jianjun Wang the leaf color of roadside camphor trees in Center of Seeding Breeding, Forestry Bureau of Ningbo, Ningbo 315012, Haishu District, Ningbo, Zhejiang Province. Zhejiang, China; and Nurturing Station for the State Key Laboratory of Seeds from two superior trees were collected Subtropical Silviculture, Institute of Biotechnology, College of Forestry and to raise seedlings in the following year. In May Biotechnology, Zhejiang Agriculture and Forestry University, Lin’an, 1999, colors of the leaf, branch, and trunk were checked against the Pantone interna- Hangzhou 311300, Zhejiang, China tional color card C (Pantone Inc., 2008). A Wangshu Zhang seedling was found obviously different from the others for its golden yellow (Pantone Center of Seeding Breeding, Forestry Bureau of Ningbo, Ningbo 315012, 101C) leaves and red (Pantone 192C) Zhejiang, China branches and trunk, and asexual propagation 1 was then conducted. It has been found that Huahong Huang these color traits are stable on the basis of Nurturing Station for the State Key Laboratory of Subtropical Silviculture, 9-year observation. In addition, a special elec- Institute of Biotechnology, College of Forestry and Biotechnology, Zhejiang trophoresis band was discovered through an Agriculture and Forestry University, Lin’an, Hangzhou 311300, Zhejiang, intersimple sequence repeat (ISSR) analysis, China suggesting that the clone was different from the others in genomic DNA (Wang et al., Additional index words.