IN THIS ISSUE Special Section Drug Development in Emerging Regions Part 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hermitage Magazine 27 En.Pdf
VIENNA / ART DECO / REMBRANDT / THE LANGOBARDS / DYNASTIC RULE VIENNA ¶ ART DECO ¶ THE LEIDEN COLLECTION ¶ REMBRANDT ¶ issue № 27 (XXVII) THE LANGOBARDS ¶ FURNITURE ¶ BOOKS ¶ DYNASTIC RULE MAGAZINE HERMITAGE The advertising The WORLD . Fragment 6/7 CLOTHING TRUNKS OF THE MUSEUM WARDROBE 10/18 THE INTERNATIONAL ADVISORY BOARD OF THE STATE HERMITAGE Game of Bowls 20/29 EXHIBITIONS 30/34 OMAN IN THE HERMITAGE The State Hermitage Museum, St. Petersburg. Inv. № ГЭ 9154 The State Hermitage Museum, St. Petersburg. Inv. Henri Matisse. 27 (XXVII) Official partners of the magazine: DECEMBER 2018 HERMITAGE ISSUE № St. Petersburg State University MAGAZINE Tovstonogov Russian State Academic Bolshoi Drama Theatre The Hermitage Museum XXI Century Foundation would like to thank the project “New Holland: Cultural Urbanization”, Aleksandra Rytova (Stella Art Foundation, Moscow) for the attention and friendly support of the magazine. FOUNDER: THE STATE HERMITAGE MUSEUM Special thanks to Svetlana Adaksina, Marina Antipova, Elena Getmanskaya, Alexander Dydykin, Larisa Korabelnikova, Ekaterina Sirakonyan, Vyacheslav Fedorov, Maria Khaltunen, Marina Tsiguleva (The State Hermitage Museum); CHAIRMAN OF THE EDITORIAL BOARD Swetlana Datsenko (The Exhibition Centre “Hermitage Amsterdam”) Mikhail Piotrovsky The project is realized by the means of the grant of the city of St. Petersburg EDITORIAL: AUTHORS Editor-in-Chief Zorina Myskova Executive editor Vladislav Bachurov STAFF OF THE STATE HERMITAGE MUSEUM: Editor of the English version Simon Patterson Mikhail Piotrovsky -

Neerlandica Extra Muros. Jaargang 44
Neerlandica extra Muros. Jaargang 44 bron Neerlandica extra Muros. Jaargang 44. Rozenberg Publishers, Amsterdam 2006 Zie voor verantwoording: http://www.dbnl.org/tekst/_nee005200601_01/colofon.php © 2015 dbnl i.s.m. 1 [Neerlandica extra Muros - februari 2006] Sera de Vriendt ........... Enkele kenmerken van het Brussels Vlaams 1. Inleiding Brussel is niet alleen de hoofdstad van België. Het is ook, naast Vlaanderen en Wallonië, een van 's lands drie gewesten. Dit gewest (Brussel in de ruime betekenis van het woord) bestaat uit negentien gemeenten, Brussel (stricto sensu) plus achttien voorsteden, en telt ongeveer een miljoen inwoners. Wat meer dan een vierde van dit totaal aantal inwoners is buitenlander: volgens R. Janssens (2001) 28.5%, waarvan 14.65% uit EU-landen en 13.86% uit niet-EU-landen. Het talrijkst zijn de Marokkanen, gevolgd door de Fransen, Italianen, Spanjaarden, Turken, Portugezen, etc. De studie van Janssens bevat tal van interessante gegevens over de talen die in Brussel gesproken worden. We beperken ons hier tot de twee voornaamste landstalen, het Frans en het Nederlands (het ligt voor de hand dat de buitenlanders ook hun eigen taal spreken, Arabisch, Berbers, Spaans, etc.; heel wat Brusselaars spreken ook Engels). Van de ondervraagde inwoners (al dan niet Belgen) verklaarde 95.6% goed tot uitstekend Frans te spreken. Slechts een derde, 33.3%, verklaarde goed tot uitstekend Nederlands te spreken. Hierbij kunnen de volgende opmerkingen gemaakt worden: - Om allerlei redenen moeten deze cijfers enigszins gerelativeerd worden. Het gaat om ‘gerapporteerde kennis’ en het is dus niet uitgesloten dat de ene milder en de ander strenger geweest is in zijn evaluatie van wat een taal ‘goed spreken’ is. -

Holding the Museum in the Palm of Your Hand Susan Hazan
Holding the Museum in the Palm of your Hand Susan Hazan Introduction: the quintessence of the museum Google Art Project and Europeana: background Transmitting tangibility; the essence of the embodied gallery and the physical object Disseminating intangibility; the descriptive qualities of textural metadata Web 1.0 versus Web 2.0 scenarios Conclusion: the loss and the gain Introduction: the quintessence of the museum When we visit a library or archive, we typically expect to find printed material, books, publications and documents. However, when we go to a museum – either in person or online – we expect a very different kind of experience. The physical museum invites us to discover exceptional and often extraordinary kinds of objects, and accordingly, when these very same objects are delivered online, they are managed very differently from the way books are managed by libraries, or the way that archives manage hierarchal documents. As the footprint of the physical museum, an online museum is therefore orchestrated to convey the singular and often spectacular nature of the objects, as well as the very quintessence of the physical museum. This means that as objects, and works of art make their screen debut, the website needs to communicate not only the physicality of the objects but also to signify - in some way - the embodied space of the gallery. As if we have just passed through the physical front door of the museum, the electronic portal signifies entrance to the online museum, setting up the collections accordingly. Objects are not simply displayed as clutches of atomized objects, but are arranged in thematic order – as a collection or exhibition – according to a chronological logic, historical narrative, provenance, or according to artists or schools of art, just in the same way that they are presented in the physical museum1. -

Best Landmarks in Amsterdam"
"Best Landmarks in Amsterdam" Created by: Cityseeker 20 Locations Bookmarked De Nieuwe Kerk "Spectacular Architecture" The Nieuwe Kerk is a 15th-century building, partly destroyed and refurbished after several fires. Located in the bustling Dam Square area of the city, this historic church has held a prominent place in the country's political and religious affairs over the centuries. It has been the venue for coronations of kings and queens, and also plays host to an array of by Dietmar Rabich exhibitions, concerts and cultural events. Admire its Gothic architecture, splendid steeples, glass-stained windows and ornate detailing. +31 20 638 6909 www.nieuwekerk.nl [email protected] Dam Square, Amsterdam Royal Palace of Amsterdam "The Royal Residence" Amsterdam's Royal Palace is the crown jewel of the city's cache of architectural marvels from the Dutch Golden Age. The palace was originally constructed in the 17th Century as the new Town Hall, designed by Jacob van Campen as a symbol of the Netherlands' far-reaching influence and its hefty stake in global commerce at that time. The palace by Diego Delso is an embodiment of opulence and lavish taste, generously adorned with marble sculptures, vivid frescoes and sparkling chandeliers that illuminate rooms of palatial proportions. Within, are numerous symbolic representations of the country's impressive economic and civic power in the realm of world politics in the 17th Century, including a larger-than-life statue of Atlas. In 1806, Louis Napoleon, brother of Napoleon Bonaparte, was named King Louis I of Holland, transforming the former Town Hall into his Royal Palace. -

Jaarverslag Kunst Kopen Met Ronald De Leeuw Puberen in De 17E Eeuw
OOG Tijdschrift van het Rijksmuseum nr.1/2008 Rijksmuseum het van Tijdschrift Oog in oog met kunst en geschiedenis OTijdschrift van het Rijksmuseumog nr.1/2008 PIERRE BOKMA ‘IK KIJK NIET MEER, IK ZIE’ Jaarverslag Kunst kopen met Ronald de Leeuw Puberen in de 17e eeuw VAN TEGENWOORDIG2007 2e JAARGANG nr.1/2008 € 5,75 1031114_coverdefnwZB.indd 1 29-01-08 17:43:48 1 Jaarverslag 2007 i Het Nieuwe Rijksmuseum: vergunningen luiden nieuwe fase in Op 13 december 2007 werd in de Philipsvleugel van het Rijks- gemeentelijke commissie voor Welstand en Monumenten is de Het museumgebouw en de tuin geboord. Daar lopen straks onzichtbaar de leidingen die het Rijksmuseum Amsterdam, het Instituut Collectie Nederland en museum de tentoonstelling Karel du Jardin geopend. Niet monumentenvergunning in 2007 verleend. Tegelijkertijd gaf het Meer dan 950.000 bezoekers kwamen in 2007 naar de presenta- klimaat in het museum regelen met lucht van de juiste tempe- de Universiteit van Amsterdam alle aanwezige kennis op het Het Nieuwe alleen hoofddirecteur Ronald de Leeuw hield een toespraak. Dagelijks Bestuur van Oud-Zuid ook de milieuvergunning af. Die tie in de Philipsvleugel van De Meesterwerken. Daar worden tijdens ratuur en vochtigheidsgraad. Die wordt aangevoerd vanuit een gebied van restauratie en conservering van (kunst)voorwerpen. Ook Egbert de Vries, voorzitter van het Dagelijks Bestuur van regelt aan welke voorwaarden het bouwplan moet voldoen op het de sluiting van het hoofdgebouw de topstukken uit de Gouden energiering die ondergronds rond het hoofdgebouw loopt. Cruz y Ortiz hebben vaak ontwerpen gemaakt voor bestaan- het Amsterdamse stadsdeel Oud-Zuid, kwam aan het woord. -
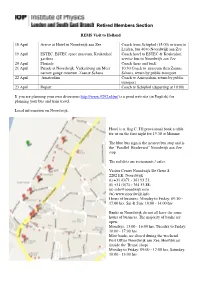
Retired Members Section
Retired Members Section REMS Visit to Holland 18 April Arrive at Hotel in Noordwijk aan Zee Coach from Schiphol (15:00) or train to Leiden, bus 40 to Noordwijk aan Zee 19 April ESTEC, ESTEC space museum, Keukenhof Coach hotel to ESTEC & Keukenhof, gardens service bus to Noordwijk aan Zee 20 April Floriade Coach there and back 21 April Parade at Noordwijk, Valkenburg am Meer 10.30 Coach to museum then Zaanse narrow gauge museum Zaanse Schans Schans, return by public transport 22 April Amsterdam Coach to Amsterdam, return by public transport 23 April Depart Coach to Schiphol (departing at 10:00) If you are planning your own diversions http://www.9292.nl/en/ is a good web site (in English) for planning your bus and train travel. Local information on Noordwijk. Hotel is at flag C. I’ll provisional book a table for us on the first night for 19.30 at Mimmo. The blue bus sign is the nearest bus stop and is the “Parallel Boulevard” Noordwijk aan Zee stop. The red dots are restaurants / cafes. Visitor Center Noordwijk De Grent 8 2202 EK Noordwijk (t) +31 (0)71 - 361 93 21; (f) +31 (0)71 - 361 53 88; (e) [email protected] (w) www.noordwijk.info Hours of business: Monday to Friday: 09:30 - 17:00 hrs, Sat & Sun: 10:00 - 14:00 hrs Banks in Noordwijk do not all have the same hours of business. The majority of banks are open: Mondays: 13:00 - 16:00 hrs, Tuesday to Friday: 10:00 - 17:00 hrs Most banks are closed during the weekend. -

NH CARANSA **** Rembrandtplein 19 1017 CV Amsterdam ESCRS
NH MUSICA **** RAI 9DQ/HLMHQEHUJKODDQ 1082 GG Amsterdam ESCRS RATE 2013 Single room € 246.00 Double/twin room € 267.00 (Breakfast and tax included) 'LVWDQFHWRFRQJUHVVFHQWHU%\WD[LNP PLQ By public transport: 15 mins The ultra-modern NH Musica hotel in Amsterdam is located in the KHDUWRIWKH=XLGDVEXVLQHVVGLVWULFWMXVWDVWRQH¶VWKURZIURPWKH RAI. The NH Musica has 213 luxurious guest rooms and all feature contemporary design, plenty of natural light and a long list of modern amenities. NH Musica’s boutique-style facility offers guest HYHU\OX[XU\LPDJLQDEOHIURPWKHVDXQDVRODULXPDQG¿WQHVV center to the sleek bar and posh Restaurant Sinoforia Nearest metro/train/bus station:9DQ1LMHQURGHZHJ 7KLVVWRSLVWKHFORVHVWE\WKHKRWHODQGZLOOWDNH\RXGLUHFWO\ WRWKH5$,E\PHWUROLQH NH CARANSA **** CC Rembrandtplein 19 1017 CV Amsterdam ESCRS RATE 2013 Single room € 239.00 Double/twin room € 258.00 (Breakfast and tax included) Distance to congress center: By taxi: 5 km (15 mins) %\SXEOLFWUDQVSRUWPLQWUDP :DONLQJPLQ The NH Caransa hotel in Amsterdam is located in the very center the city on Rembrandt Square, which is considered the heart of Amsterdam nightlife. With its excellent location, the modern and spacious NH Caransa hotel offers a total of 66 stylish and contemporary Standard and Superior rooms. Refreshing color tones, soundproof windows, luxuriously thick mattresses, a VHOHFWLRQRIFRPIRUWDEOHSLOORZVDQGSULYDWHEDWKURRPVDUHMXVW some of the features of our guest rooms. Nearest metro/train/bus station:5HPEUDQGWSOHLQ 2SSRVLWHWKHKRWHOLVWKHWUDPVWRSIRUOLQHWDNLQJ\RXLQ 2QHVWUDLJKWZD\WRWKHFRQJUHVVFHQWHU Kuoni Destination Management Benelux | Teleport Boulevard 110 | NL- 1043 EJ Amsterdam, the Netherlands Phone: +31 20 627 00 60 | Fax: +21 20 422 69 69 | E-mail [email protected]. -

NGA | 2017 Annual Report
N A TIO NAL G ALL E R Y O F A R T 2017 ANNUAL REPORT ART & EDUCATION W. Russell G. Byers Jr. Board of Trustees COMMITTEE Buffy Cafritz (as of September 30, 2017) Frederick W. Beinecke Calvin Cafritz Chairman Leo A. Daly III Earl A. Powell III Louisa Duemling Mitchell P. Rales Aaron Fleischman Sharon P. Rockefeller Juliet C. Folger David M. Rubenstein Marina Kellen French Andrew M. Saul Whitney Ganz Sarah M. Gewirz FINANCE COMMITTEE Lenore Greenberg Mitchell P. Rales Rose Ellen Greene Chairman Andrew S. Gundlach Steven T. Mnuchin Secretary of the Treasury Jane M. Hamilton Richard C. Hedreen Frederick W. Beinecke Sharon P. Rockefeller Frederick W. Beinecke Sharon P. Rockefeller Helen Lee Henderson Chairman President David M. Rubenstein Kasper Andrew M. Saul Mark J. Kington Kyle J. Krause David W. Laughlin AUDIT COMMITTEE Reid V. MacDonald Andrew M. Saul Chairman Jacqueline B. Mars Frederick W. Beinecke Robert B. Menschel Mitchell P. Rales Constance J. Milstein Sharon P. Rockefeller John G. Pappajohn Sally Engelhard Pingree David M. Rubenstein Mitchell P. Rales David M. Rubenstein Tony Podesta William A. Prezant TRUSTEES EMERITI Diana C. Prince Julian Ganz, Jr. Robert M. Rosenthal Alexander M. Laughlin Hilary Geary Ross David O. Maxwell Roger W. Sant Victoria P. Sant B. Francis Saul II John Wilmerding Thomas A. Saunders III Fern M. Schad EXECUTIVE OFFICERS Leonard L. Silverstein Frederick W. Beinecke Albert H. Small President Andrew M. Saul John G. Roberts Jr. Michelle Smith Chief Justice of the Earl A. Powell III United States Director Benjamin F. Stapleton III Franklin Kelly Luther M. -

Mijn Rembrandt
VAN DE MAKERS VAN HET NIEUWE RIJKSMUSEUM DISCOURS FILM PRESENTEERT MIJN REMBRANDT EEN FILM VAN OEKE HOOGENDIJK PERSMAP MIJN REMBRANDT NEDERLAND | 95 MINUTEN | KLEUR EEN PRODUCTIE VAN Discours Film Oeke Hoogendijk & Frank van den Engel MET STEUN VAN Nederlands Filmfonds Production Incentive AFK Fonds 21 VSB Fonds PRODUCENT Discours Film Oeke Hoogendijk, Frank van den Engel +31(0)6 51 17 00 99 [email protected] DISTRIBUTEUR BENELUX Cinéart Nederland B.V. T +31(0)20 5308848 www.cineart.nl PERS Julia van Berlo M: +31 (0)6 83785238 T: +31 (0)20 5308840 [email protected] SALES & DISTRIBUTIE Cinephil – Philippa Kowarsky Cinephil.com [email protected] MIJN REMBRANDT PERSMAP 2 MIJN REMBRANDT LOGLINE Rembrandt houdt, 350 jaar na zijn dood, de kunst- wereld nog altijd in zijn greep. De epische documen- taire MIJN REMBRANDT duikt in de exclusieve wereld van Rembrandtbezitters en Rembrandtjagers, waar men gedreven wordt door bewondering, hoogmoed, geld en afgunst, maar bovenal door een grenzeloze passie en liefde voor het werk van de grootmeester van de intimiteit. MIJN REMBRANDT PERSMAP 3 SYNOPSIS MIJN REMBRANDT speelt zich af in de wereld van de oude meesters en biedt een mozaïek van aangrijpende verhalen, waarin de grenzeloze passie voor Rembrandts schilderijen leidt tot dramatische ontwik- kelingen en verrassende plotwendingen. Terwijl collectioneurs zoals het echtpaar De Mol van Otterloo, de Amerikaan Thomas Kaplan en de Schotse hertog van Buccleuch, ons deelgenoot maken van hun speciale band met ‘hun’ Rembrandt, brengt de Franse baron Eric de Rothschild twee Rembrandts op de markt. Dit is het begin van een verbeten politieke strijd tussen het Rijksmuseum en het Louvre. -

Saskia, De Vrouw Van Rembrandt
Saskia, de vrouw van Rembrandt 120256_p001_160.indd 1 17-09-12 10:56 120256_p001_160.indd 2 17-09-12 10:56 Saskia De vrouw van Rembrandt ben broos 120256_p001_160.indd 3 17-09-12 10:56 4 120256_p001_160.indd 4 17-09-12 10:56 inhoud voorwoord 6 het leven van saskia, de vrouw van rembrandt 9 i. rombertus uylenburgh (1554-1624) 12 ii. de broers en zusters van saskia 16 iii. gerrit van loo (ca. 1583-1641) 19 iv. johannes maccovius (1588-1644) 22 v. hendrick uylenburgh (1584/1589-1661) 24 vi. rembrandt (1606-1669) 26 vii. aeltje uylenburgh (1570-1644) 29 viii. françois coopal (1600-1658/1659) 35 ix. sint annaparochie door rembrandt getekend (1633) 36 x. het ‘album ockema’ 44 xi. johannes cornelisz. sylvius (1564-1638) 50 xii. govaert flinck (1615-1660) 60 xiii. antje van loo (1635-?) en aeltje van wijck (1637-na 1662), de petekinderen van saskia 66 xiv. petrus sylvius (1610-1653) 84 saskia galerij 104 saskia kroniek 116 saskia en het sprookje van houbraken 128 saskia en haar familie: genealogie i en ii 146 bibliografie 150 index 154 nawoord 158 colofon 160 120256_p001_160.indd 5 17-09-12 10:56 voorwoord In 1893 verscheen in Amerika het boekje Saskia. The Wife of Rembrandt van Charles Knowles Bolton, een bibliothecaris te Boston. (afb. 109a) Het was een gloedvol levensverhaal, geschreven aan de hand van archiefstukken over Saskia Uylenburgh. Die waren veertig jaar eerder - dat heette toen recent - teruggevonden door de Nederlandse archivarissen Pieter Scheltema in Amsterdam en Wopke Eekhoff in Leeuwarden. De schrij ver bleek zij n geestdrift geërfd te hebben van zij n moeder, die een vrouwenactiviste was en populariteit genoot als biografe van beroemde vrouwelij ke tij dgenoten. -

Thomas Kaplan, La Passion De Rembrandt
Thomas Kaplan, la passion de Rembrandt Le milliardaire américain, qui possède de nombreuses œuvres du maître, offre une toile de Ferdinand Bol au Louvre. LE MONDE | 02.01.2017 à 06h41 • Mis à jour le 10.01.2017 à 16h42 |Par Roxana Azimi « Parler de Rembrandt me transporte », avoue Thomas Kaplan, sans naïveté mais avec une candeur revendiquée, nullement émoussée par quatre siècles d’exégèses savantes. Habituellement, l’homme d’affaires américain est invité à s’exprimer sur des sujets sérieux : le cours des métaux précieux, dans lesquels il a fait fortune ; le terrorisme, qui l’a poussé à créer, en 2012 à Harvard, un programme à l’intention des futurs pontes des services secrets ; ou encore les félins, qu’il s’échine à protéger. Cette fois, il change de registre, comblé à l’idée d’exposer les onze Rembrandt (1606-1669) de sa collection personnelle au Musée du Louvre, à Paris, aux côtés de dix-neuf autres tableaux des élèves du maître, à partir du 22 février. « C’est la première fois que je les verrai réunis ! », dit-il avec gourmandise. Cet épicurien de 54 ans, amateur de cigares et de rosé, ne traite pas pour autant de l’art avec légèreté. Il y va du sens de la vie. Voilà encore treize ans, Thomas Kaplan ne se voyait guère dans la peau d’un collectionneur. Sous l’impulsion de son épouse, Daphné, il s’était certes passionné pour le design des années 1950, en particulier Carlo Mollino. Pour autant, acheter de l’art ne l’effleurait guère. « Ma priorité, c’était de promouvoir certains principes de vie, se justifie-t-il. -

Observaties Rembrandtplein
Observaties Rembrandtplein Verkenning van de bezoekersstromen en het functioneren van de huidige festivalaanpak in het uitgaansgebied Rembrandtplein DSP-groep - Paul van Soomeren en Randy Bloeme Gemeente Amsterdam OOV - Pieter Walinga en Marek Kruszel / Dewi Zloch Verkenning van de bezoekersstromen en het functioneren van de huidige festivalaanpak in het uitgaansgebied Rembrandtplein Onderzoeksmethoden Observaties door onderzoekers (met achtergrondkennis van eerdere observaties in november 2015), maar met name door observanten uit de festival wereld. zaterdag 4 juni – vrijdag 10 juni – vrijdag 17 juni, vrijdag 22 juli, vrijdag 29 juli Deskresearch (op basis van onderzoeken en beleidstukken; aangeleverd door opdrachtgever). Brainstorm met onderzoekers en observatoren Focus van het onderzoek Aandacht voor de rol van voorzieningen, routing/verwijzingen en logistieke aan-/afvoer Globaal overzicht van bezoekersstromen (zie bijlagen) alsmede ‘verblijfstellingen op de pleinen’ Inzicht in ontwikkeling van de uitgaansnacht; o.a. probleemgedrag Zijdeling in laatste observatie meegenomen: afval en vervuiling Bijlagen Schema verbetervoorstellen Probleem- en oplossingenkaart Tellingen bezoekersstromen DSP-groep RAPPORT ─ Observaties Rembrandtplein 2 Inhoud 1 Uitkomsten en verbetervoorstellen 4 2 Logistieke aan-/afvoer 5 2.1 Autoverkeer 5 2.2 Fietsverkeer 6 2.3 Voetgangers 6 2.4 Conclusies, adviezen en nadere verkenning 6 3 Voorzieningen 8 3.1 Conclusies, adviezen en nadere verkenning 9 4 Communicatie 11 4.1 Fysieke communicatie 11 4.2 Sociale