Acral Acquired Cutis Laxa Associated with Iga Multiple Myeloma, Joint Hyperlaxity and Urticarial Neutrophilic Dermatosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
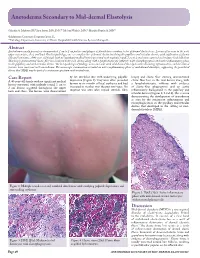
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years. -

De Barsy Syndrome
orphananesthesia Anesthesia recommendations for patients suffering from De Barsy syndrome Disease name: De Barsy syndrome ICD 10: Q87.7; OMIM 614438 Synonyms: DBS, De Barsy-Moens-Dierckx syndrome, Progeroid syndrome of De Barsy, Autosomal recessive cutis laxa Type 3 *With 2 gene subdivisions: ARCL3A: caused by a ALDH18A1 mutation ARCL3B: caused by a PYCR1 mutation DeBarsy syndrome is a rare clinical syndrome characterized by cutis laxa, ophthalmic opacification, skeletal malformations, as well as mental and growth retardation. This disease is genetically transmitted in an autosomal recessive fashion. Affected patients often require surgical correction of ophthalmic and orthopaedic abnormalities. This syndrome was first described by A.M. De Barsy in 1967 and less than 100 known cases are documented in the medical literature. Very little has been published on this rare disorder and only a single article has addressed anesthesia case outcomes and management strategies (Aponte, 2010). The diverse collection of clinical manifestations in De Barsy syndrome includes: intra-uterine growth retardation (IUGR), postnatal growth delay, motor delay, cognitive impairment, hypontonia, athetoid movements, malformations, microcephaly, wormian bones, large fontanelles, facial dysmorphism, cataracts, corneal clouding, thin/wrinkled skin, easy bruising, sparse hair, joint laxity, osteopenia, and inguinal hernias. Medicine in progress Perhaps new knowledge Every patient is unique Perhaps the diagnostic is wrong Find more information on the disease, its centres -

Dermatologists Cannot Be Lax About Acquired Cutis Laxa by Warren R
Dermatologists cannot be lax about acquired cutis laxa By Warren R. Heymann, MD Jan. 28, 2019 Acral localized acquired cutis laxa. Loose, redundant skin on the volar finger pads bilaterally that gave them a flat, rounded appearance. Credit: JAADFrom the time I was a medical student, I have always been fascinated by the “little old man” appearance of patient with cutis laxa (CL). In their excellent review, Berk et al note that CL is characterized by abnormal elastic fibers resulting in loose, redundant skin. Typically, skin in CL can easily be pulled and only slowly returns to its original position. Unlike some conditions in the differential diagnosis, notably Ehlers-Danlos syndrome(s) or pseudoxanthoma elasticum, easy bruising or abnormal scarring does not characterize CL. Redundant skin is often most noticeable on the neck, hands, and groin, but can also be seen on the face, creating a premature aging appearance. CL may be inherited or acquired (ACL). Inherited forms include autosomal dominant CL; autosomal recessive CL (ARCL)-I, -IIA, and -IIB; X-linked CL and other variants. Although all of the inherited forms of CL are rare, ARCL has most commonly been reported, particularly ARCL- II. Because of significant overlap among these types, precise clinical classification can be difficult. For the clinical differentiation of the variants and their genetic mutations, please refer to Berk et al (1). The etiology of CL involves abnormalities the elastic fiber network in skin and internal organs. Autosomal dominant CL has been associated with mutations of the elastin gene. X-linked CL is associated with gene mutations ATPA7 and abnormalities of copper transport. -
The Skin in Genetically-Controlled Metabolic Disorders P
Review Article J Med Genet: first published as 10.1136/jmg.10.2.101 on 1 June 1973. Downloaded from Journal of Medical Genetics (1973). 10, 101. The Skin in Genetically-controlled Metabolic Disorders P. C. H. NEWBOLD Department of Medicine, Cambridge University Medical School, Hills Road, Cambridge CB2 2QL Diseased nature oftentimes breaks forth however, it may lead to difficulty in assessing intelli- In strange eruptions.-Henry IV, part 1, III, i. gence, which is within normal range in 50% of The skin is now commonly accepted as a mirror patients. There is suggestive evidence of a re- of internal disease, but as with other looking-glasses, lationship between subnormal folate levels and low the evidence offered may be selected or ignored. intelligence (Carey et al, 1968), and studies have The epidermis is an interesting structure, especially shown increased turnover of folate coenzymes and rich in tyrosine, phenylalanine, tryptophan, and resulting folate depletion in these patients (Carey et histidine, when compared with the corium (Roth- al, 1968; Butterworth, Krumdieck, and Baugh, man, 1965). Tyrosinaemia and histidinaemia do 1971). There is also a high incidence of an organic not include cutaneous manifestations, but culture of brain syndrome following intracranial vascular skin fibroblasts is a helpful diagnostic tool for study- thromboses (Dunn, Perry, and Dolman, 1966), ing metabolic defects such as citrullinaemia, cysti- copyright. nosis, and maple-syrup urine disease (Scriver, 1969). Most of the conditions now to be described are rare, but if these metabolic diseases were com- mon, there could be no human race as we know it. Homocystinuria http://jmg.bmj.com/ This most informative anomaly was discovered during a study of mentally retarded patients in Ire- land (Carson and Neill, 1962). -

RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa
REPORT RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa, and Scoliosis: MACS Syndrome Lina Basel-Vanagaite,1,2,14 Ofer Sarig,4,14 Dov Hershkovitz,6,7 Dana Fuchs-Telem,2,4 Debora Rapaport,3 Andrea Gat,5 Gila Isman,4 Idit Shirazi,4 Mordechai Shohat,1,2 Claes D. Enk,10 Efrat Birk,2 Ju¨rgen Kohlhase,11 Uta Matysiak-Scholze,11 Idit Maya,1 Carlos Knopf,9 Anette Peffekoven,12 Hans-Christian Hennies,12 Reuven Bergman,8 Mia Horowitz,3 Akemi Ishida-Yamamoto,13 and Eli Sprecher2,4,6,* Inherited disorders of elastic tissue represent a complex and heterogeneous group of diseases, characterized often by sagging skin and occasionally by life-threatening visceral complications. In the present study, we report on an autosomal-recessive disorder that we have termed MACS syndrome (macrocephaly, alopecia, cutis laxa, and scoliosis). The disorder was mapped to chromosome 20p11.21-p11.23, and a homozygous frameshift mutation in RIN2 was found to segregate with the disease phenotype in a large consan- guineous kindred. The mutation identified results in decreased expression of RIN2, a ubiquitously expressed protein that interacts with Rab5 and is involved in the regulation of endocytic trafficking. RIN2 deficiency was found to be associated with paucity of dermal micro- fibrils and deficiency of fibulin-5, which may underlie the abnormal skin phenotype displayed by the patients. Disorders of elastic tissue often share common phenotypic shown to result in decreased secretion of elastin.9,10 In features, including redundant skin, hyperlaxity of joints, addition, -

Genetics of Lipedema: New Perspectives on Genetic Research and Molecular Diagnoses S
European Review for Medical and Pharmacological Sciences 2019; 23: 5581-5594 Genetics of lipedema: new perspectives on genetic research and molecular diagnoses S. PAOLACCI1, V. PRECONE2, F. ACQUAVIVA3, P. CHIURAZZI4,5, E. FULCHERI6,7, M. PINELLI3,8, F. BUFFELLI9,10, S. MICHELINI11, K.L. HERBST12, V. UNFER13, M. BERTELLI2; GENEOB PROJECT 1MAGI’S LAB, Rovereto (TN), Italy 2MAGI EUREGIO, Bolzano, Italy 3Department of Translational Medicine, Section of Pediatrics, Federico II University, Naples, Italy 4Istituto di Medicina Genomica, Fondazione A. Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy 5UOC Genetica Medica, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy 6Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 7Department of Integrated Surgical and Diagnostic Sciences, University of Genoa, Genoa, Italy 8Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli, Italy 9Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 10Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal-Infantile Sciences, University of Genoa, Genoa, Italy 11Department of Vascular Rehabilitation, San Giovanni Battista Hospital, Rome, Italy 12Department of Medicine, University of Arizona, Tucson, AZ, USA 13Department of Developmental and Social Psychology, Faculty of Medicine and Psychology, Sapienza University of Rome, Rome, Italy Abstract. – OBJECTIVE: The aim of this quali- Introduction tative review is to provide an update on the cur- rent understanding of the genetic determinants of lipedema and to develop a genetic test to dif- Lipedema is an underdiagnosed chronic debil- ferentiate lipedema from other diagnoses. itating disease characterized by bruising and pain MATERIALS AND METHODS: An electronic and excess of subcutaneous adipose tissue of the search was conducted in MEDLINE, PubMed, and legs and/or arms in women during or after times Scopus for articles published in English up to of hormone change, especially in puberty1. -

Acquired Cutis Laxa of Face with Multiple Myeloma
Letters to the Editor 6. Takayama K, Satoh T, Hayashi M, Yokozeki H. Psoriatic skin Serum β2‑microglobulin was increased. Bone marrow lesions induced by BCG vaccination. Acta Derm Venereol examination revealed 14% plasma cells. Based on 2008;88:621‑2. 7. Monacelli M. Koebner reaction in psoriasis. In: Farber EM, these findings, a diagnosis of early multiple myeloma Cox AJ, editors. Psoriasis: Proceedings of the International with nephrotic syndrome was made. She was started Symposium. Vol. 99. Stanford: Stanford University press; 1971. p. 104‑11. on a chemotherapy regimen consisting of thalidomide 8. Rambukkana A, Das PK, Witkamp L, Yong S, Meinardi MM, (100 mg/day), oral cyclophosphamide (150 mg/day) Bos JD. Antibodies to mycobacterial 65‑kDa heat shock protein both for the first five days in every month with weekly and other immunodominantantigens in patients with psoriasis. J Invest Dermatol 1993;100:87‑92. intravenous dexamethasone (40 mg/week) along with loop diuretics. In addition, the patient was prescribed Access this article online bortezomib which was discontinued by the patient after two months. Following one year of treatment, the Quick Response Code: Website: www.ijdvl.com anasarca subsided leaving behind loose wrinkled skin all over the body. Over the next one year, the skin over the DOI: 10.4103/0378-6323.140309 extremities and the abdomen reverted back to normal; however, loosening of skin persisted over the face. PMID: ***** Examination revealed a healthy‑appearing woman, who appeared older than her stated age [Figure 1]. She Acquired cutis laxa of face with had loose, sagging skin on the face and neck giving her a hound‑dog facies [Figure 2]. -

Table I. Genodermatoses with Known Gene Defects 92 Pulkkinen
92 Pulkkinen, Ringpfeil, and Uitto JAM ACAD DERMATOL JULY 2002 Table I. Genodermatoses with known gene defects Reference Disease Mutated gene* Affected protein/function No.† Epidermal fragility disorders DEB COL7A1 Type VII collagen 6 Junctional EB LAMA3, LAMB3, ␣3, 3, and ␥2 chains of laminin 5, 6 LAMC2, COL17A1 type XVII collagen EB with pyloric atresia ITGA6, ITGB4 ␣64 Integrin 6 EB with muscular dystrophy PLEC1 Plectin 6 EB simplex KRT5, KRT14 Keratins 5 and 14 46 Ectodermal dysplasia with skin fragility PKP1 Plakophilin 1 47 Hailey-Hailey disease ATP2C1 ATP-dependent calcium transporter 13 Keratinization disorders Epidermolytic hyperkeratosis KRT1, KRT10 Keratins 1 and 10 46 Ichthyosis hystrix KRT1 Keratin 1 48 Epidermolytic PPK KRT9 Keratin 9 46 Nonepidermolytic PPK KRT1, KRT16 Keratins 1 and 16 46 Ichthyosis bullosa of Siemens KRT2e Keratin 2e 46 Pachyonychia congenita, types 1 and 2 KRT6a, KRT6b, KRT16, Keratins 6a, 6b, 16, and 17 46 KRT17 White sponge naevus KRT4, KRT13 Keratins 4 and 13 46 X-linked recessive ichthyosis STS Steroid sulfatase 49 Lamellar ichthyosis TGM1 Transglutaminase 1 50 Mutilating keratoderma with ichthyosis LOR Loricrin 10 Vohwinkel’s syndrome GJB2 Connexin 26 12 PPK with deafness GJB2 Connexin 26 12 Erythrokeratodermia variabilis GJB3, GJB4 Connexins 31 and 30.3 12 Darier disease ATP2A2 ATP-dependent calcium 14 transporter Striate PPK DSP, DSG1 Desmoplakin, desmoglein 1 51, 52 Conradi-Hu¨nermann-Happle syndrome EBP Delta 8-delta 7 sterol isomerase 53 (emopamil binding protein) Mal de Meleda ARS SLURP-1 -

Blueprint Genetics Progeria and Progeroid Syndromes Panel
Progeria and Progeroid Syndromes Panel Test code: DE0201 Is a 17 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of Hutchinson-Gilford progeria syndrome or a syndrome with progeroid features. About Progeria and Progeroid Syndromes Hutchinson-Gilford progeria syndrome (HGPS) is caused by LMNA mutations with autosomal dominant inheritance but almost all individuals with HGPS have a de novo mutation. All the major syndromes with proreroid features have autosomal recessive inheritance although the ALDH18A1 related cutis laxa is also inherited in dominant manner. HGPS manifest with features of accelerated aging observed in early childhood. Age of disease onset and progress rate varies but is remarkably consistent overall. Children with HGPS usually appear normal at birth. Profound failure to thrive occurs during the first year. Patients have a head disproportionately large for face, prominent eyes, partial alopecia progressing to total alopecia, loss of subcutaneous fat, progressive joint contractures, bone changes and nail dystrophy occur by age of three. Later symptoms include conductive hearing loss, dental crowding and partial lack of secondary tooth eruption. Motor and mental development is normal. Average life span is approximately 15 years. Premature death occurs as a result of atherosclerotic events, either myocardial infarct or stroke. Diagnosis is based on clinical features and detection of heterozygous LMNA variants either within exon 11 (termed classic HGPS) or at the intronic border of exon 11 (termed atypical HGPS). Although no other gene than LMNA associates with HGPS, premature aging occur in many other syndromes with so-called progeroid features, thus the panel also include genes causing the following syndromes: Cockayne syndrome, congenital generalized lipodystrophy, cutis laxa and progeroid type Ehlers-Danlos syndrome among some others. -

Case Report: Acquired Cutis Laxa in a Six Year Old Girl
Case Report: Acquired Cutis Laxa in a Six Year Old Girl Ehiaghe L ANABA, FWACP, FMCP, MSc Dermpath, Adekunle O GEORGE2 FMCP, Adebola O OGUNBIYI2 FMCP, FWACP, Olabiyi G OGUN FMCPath3 1Department of Medicine, Lagos State University Teaching Hospital, Lagos, Nigeria. 2Department of Medicine, University College Hospital, Ibadan, Nigeria. 3Department of Pathology, University College Hospital, Ibadan, Nigeria. ABSTRACT Cutis laxa is a rare skin disease of unknown aetiology. Evaluation of the skin shows loss of elastic fibers with resultant laxity of the skin. We present the case of a 6 year old girl who had normal skin until age 2. She presented with a four year history of generalized pruritus, lax skin and looked older than her age. KEYWORDS: Acquired Cutis Laxa, Generalised Pruritus, Lax skin. INTRODUCTION Biopsy from the lax skin of the armpit showed paucity of elastic fibers in the dermis (Fig 4). No Cutis laxa (CL) is a rare skin disease of unknown inflammatory cells were seen in the dermis. Other aetiology with defects in elastic tissue synthesis or its investigations were normal. A diagnosis of acquired destruction.1-3 Cutis laxa can be congenital or cutis laxa was made. acquired,4--7 characterized by loose inelastic skin. Acquired cutis laxa (ACL) is rare, occurs in both 2,8 DISCUSSION children and adults, and can be generalized or localized.6,9,10 Clinically, CL manifests as lax skin and a premature aged appearance which may involve the entire skin CASE REPORT surface especially the face, neck, back and thighs.1,3,10 The skin hangs in redundant folds especially in the A 6 year old girl presented to us with a 4 year history neck and axillae as is the case with our patient.10-12 of loose skin and generalized increased skin Acquired cutis laxa can be idiopathic9,10,13 or darkening. -

TEST CATALOGUE October 2020 ACHIEVING a POSITIVE CHANGE
TEST CATALOGUE October 2020 ACHIEVING A POSITIVE CHANGE ... CENTOGENE IS DEDICATED TO TRANSFORMING THE SCIENCE OF GENETIC INFORMATION INTO SOLUTIONS AND HOPE FOR PATIENTS WITH RARE DISEASES AND THEIR FAMILIES. BOSTON We achieve this by: › Leveraging the world’s largest database in rare genetic disorders with genetic, proteomic, metabolomic, and clinical information – CentoMD® › Applying our knowledge derived from our global diagnostic testing services – addressing the worldwide heterogeneity in ethnicities › Guaranteeing the highest quality in our processes based on the highest level of accreditation OUR GLOBAL FOOTPRINT The outcome of growth and expansion at CENTOGENE is the inauguration of new locations. As a cosmopolitan company, we have a strong international footprint. ROSTOCK HAMBURG BERLIN DELHI FRANKFURT VIENNA DUBAI SOLID GROWTH IN JUST OVER A DECADE Metabolomic Oncogenetic Biomarkers MLPA >180 NGS panels platform testing CentoCard® qPCR Whole exome 2006 The logistic solution sequencing Diagnostics in neurogenetic diseases Non-invasive Carrier > 9,000 Whole genome prenatal testing screening different test sequencing 2016 (CentoNIPT®) (CentoScreen®) assays CentoMD® Proteomic Microarrays High throughput Transcriptomics The mutation platform genomic facility 2018 database iPSC program IPO Artificial Intelligence 2020+ initiative 2019 Providing world-class genetic testing and life-changing solutions for all rare disease patients 2019 WHAT DRIVES CENTOGENE Our goal: providing precise medical diagnosis of inherited diseases at the earliest possible moment; transforming medical expertise and analytical information into actionable results for physicians, patients, and pharmaceutical partners. Our commitment: life-long support for our patients and partners - driven by the continuous improvement of our diagnostic quality and therapeutic options for individual patients. Our work does not end by just delivering a medical diagnosis. -

Genetic and Developmental Disorders of the Oral Mucosa: Epidemiology; Molecular Mechanisms; Diagnostic Criteria; Management
DOI: 10.1111/prd.12261 REVIEW ARTICLE Genetic and developmental disorders of the oral mucosa: Epidemiology; molecular mechanisms; diagnostic criteria; management Roberto Pinna1 | Fabio Cocco1,2 | Guglielmo Campus1,2,3 | Giulio Conti4 | Egle Milia1 | Andrea Sardella4,5 | Maria Grazia Cagetti2,5 1Department of Surgery, Medicine and Experimental Sciences, University of Sassari, Sassari, Italy 2WHO Collaboration Centre for Epidemiology and Community Dentistry, University of Milan, Milan, Italy 3Klinik für Zahnerhaltung, Präventiv-und Kinderzahnmedizin Zahnmedizinische Kliniken (ZMK), University of Bern, Switzerland 4IRCCS “Ca Granda-Ospedale Maggiore”, University of Milan, Milan, Italy 5Department of Biomedical, Surgical and Dental Science, University of Milan, Milan, Italy Correspondence Guglielmo Campus, Prof Guglielmo Campus Klinik für Zahnerhaltung, Präventiv-und Kinderzahnmedizin Zahnmedizinische Kliniken (ZMK) University of Bern, Freiburgstrasse 7, Bern, Switzerland. Emails: [email protected]; [email protected] 1 | INTRODUCTION exact prevalence is unknown, but the syndrome seems more common among the Amish community. This rare condition is inherited as an Oral mucosal lesions may appear as ulcers, color changes and altera- autosomal recessive trait with a variable expression. Mutations of the tions in size and configuration of oral anatomy. This review presents Ellis van Creveld protein 1 and 2 genes, located in a head- to- head con- a broad overview of genetic and developmental disorders of the figuration on chromosome 4p16, have been identified as causative.6,7 oral mucosa that might be recognized in children, adults and the el- The mutations of Ellis van Creveld belong to the short rib- polydactyly derly.1-3 A number of genetic disorders (Table 1) have specific mani- group and especially type III (Verma- Naumoff syndrome).