A Clinical Study on Genodermatoses and Their Effect On
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The National Economic Burden of Rare Disease Study February 2021
Acknowledgements This study was sponsored by the EveryLife Foundation for Rare Diseases and made possible through the collaborative efforts of the national rare disease community and key stakeholders. The EveryLife Foundation thanks all those who shared their expertise and insights to provide invaluable input to the study including: the Lewin Group, the EveryLife Community Congress membership, the Technical Advisory Group for this study, leadership from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH), the Undiagnosed Diseases Network (UDN), the Little Hercules Foundation, the Rare Disease Legislative Advocates (RDLA) Advisory Committee, SmithSolve, and our study funders. Most especially, we thank the members of our rare disease patient and caregiver community who participated in this effort and have helped to transform their lived experience into quantifiable data. LEWIN GROUP PROJECT STAFF Grace Yang, MPA, MA, Vice President Inna Cintina, PhD, Senior Consultant Matt Zhou, BS, Research Consultant Daniel Emont, MPH, Research Consultant Janice Lin, BS, Consultant Samuel Kallman, BA, BS, Research Consultant EVERYLIFE FOUNDATION PROJECT STAFF Annie Kennedy, BS, Chief of Policy and Advocacy Julia Jenkins, BA, Executive Director Jamie Sullivan, MPH, Director of Policy TECHNICAL ADVISORY GROUP Annie Kennedy, BS, Chief of Policy & Advocacy, EveryLife Foundation for Rare Diseases Anne Pariser, MD, Director, Office of Rare Diseases Research, National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Elisabeth M. Oehrlein, PhD, MS, Senior Director, Research and Programs, National Health Council Christina Hartman, Senior Director of Advocacy, The Assistance Fund Kathleen Stratton, National Academies of Science, Engineering and Medicine (NASEM) Steve Silvestri, Director, Government Affairs, Neurocrine Biosciences Inc. -

Neonatal Dermatology Review
NEONATAL Advanced Desert DERMATOLOGY Dermatology Jennifer Peterson Kevin Svancara Jonathan Bellew DISCLOSURES No relevant financial relationships to disclose Off-label use of acitretin in ichthyoses will be discussed PHYSIOLOGIC Vernix caseosa . Creamy biofilm . Present at birth . Opsonizing, antibacterial, antifungal, antiparasitic activity Cutis marmorata . Reticular, blanchable vascular mottling on extremities > trunk/face . Response to cold . Disappears on re-warming . Associations (if persistent) . Down syndrome . Trisomy 18 . Cornelia de Lange syndrome PHYSIOLOGIC Milia . Hard palate – Bohn’s nodules . Oral mucosa – Epstein pearls . Associations . Bazex-Dupre-Christol syndrome (XLD) . BCCs, follicular atrophoderma, hypohidrosis, hypotrichosis . Rombo syndrome . BCCs, vermiculate atrophoderma, trichoepitheliomas . Oro-facial-digital syndrome (type 1, XLD) . Basal cell nevus (Gorlin) syndrome . Brooke-Spiegler syndrome . Pachyonychia congenita type II (Jackson-Lawler) . Atrichia with papular lesions . Down syndrome . Secondary . Porphyria cutanea tarda . Epidermolysis bullosa TRANSIENT, NON-INFECTIOUS Transient neonatal pustular melanosis . Birth . Pustules hyperpigmented macules with collarette of scale . Resolve within 4 weeks . Neutrophils Erythema toxicum neonatorum . Full term . 24-48 hours . Erythematous macules, papules, pustules, wheals . Eosinophils Neonatal acne (neonatal cephalic pustulosis) . First 30 days . Malassezia globosa & sympoidalis overgrowth TRANSIENT, NON-INFECTIOUS Miliaria . First weeks . Eccrine -

Ectodermal Dysplasias: a New Clinical-Genetic Classification
J Med Genet 2001;38:579–585 579 Ectodermal dysplasias: a new clinical-genetic J Med Genet: first published as 10.1136/jmg.38.9.579 on 1 September 2001. Downloaded from classification Manuela Priolo, Carmelo Laganà Abstract many case reports and personal communica- The ectodermal dysplasias (EDs) are a tions in their listing of EDs, as well as large and complex nosological group of conditions traditionally classified under other diseases, first described by Thurnam in headings, for example dyskeratosis congenita11 1848. In the last 10 years more than 170 and keratitis-ichthyosis-deafness (KID) syn- diVerent pathological clinical conditions drome12 (poikiloderma and immune defect have been recognised and defined as EDs, diseases and erythrokeratodermas, respec- all sharing in common anomalies of the tively). Further, they did not appear to hair, teeth, nails, and sweat glands. Many consider variability of expression and may are associated with anomalies in other have reported, as distinct diseases, conditions organs and systems and, in some condi- that reflect variable expression of the same tions, with mental retardation. pathological entity. Moreover, they included The anomalies aVecting the epidermis pathological conditions which, in our opinion, and epidermal appendages are extremely do not strictly fulfil the diagnostic criteria for variable and clinical overlap is present EDs, such as conditions with secondary among the majority of EDs. Most EDs are involvement of epidermal derivatives rather defined by particular clinical signs (for than a primary defect. We abandoned the 1-2- example, eyelid adhesion in AEC syn- 3-4 designation of EDs, because we believe drome, ectrodactyly in EEC). -

Bilateral Lower Extremity Hyperkeratotic Plaques: a Case Report of Ichthyosis Vulgaris
Faculty & Staff Scholarship 2015 Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Zachary Zinn Omid Jalali Follow this and additional works at: https://researchrepository.wvu.edu/faculty_publications Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Abstract: Here, we report a case of a middle-aged woman presenting with severe, long-standing, Zachary Zinn hyperkeratotic plaques of the lower extremities unrelieved by over-the-counter medications. Omid Jalali Initial history and clinical findings were suggestive of an inherited ichthyosis. Ichthyoses are genetic disorders characterized by dry scaly skin and altered skin-barrier function. A diagnosis Department of Dermatology, West Virginia University, of ichthyosis vulgaris was confirmed by histopathology. Etiology, prevalence, and treatment Morgantown, WV, USA options are discussed. Keywords: filaggrin gene, FLG, profilaggrin, keratohyalin granules, hyperkeratosis Introduction For personal use only. Inherited ichthyoses are a diverse group of genetic disorders characterized by dry, scaly skin; hyperkeratosis; and altered skin-barrier function. While these disorders of cutaneous keratinization are multifaceted and varying in etiology, disruption in the stratum corneum with generalized scaling is common to all.1–4 Although not entirely known -

Revertant Mosaicism in a Human Skin Fragility Disorder Results from Slipped Mispairing and Mitotic Recombination
Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi, … , Leena Bruckner-Tuderman, Cristina Has J Clin Invest. 2012;122(5):1742-1746. https://doi.org/10.1172/JCI61976. Brief Report Dermatology Spontaneous gene repair, also called revertant mosaicism, has been documented in several genetic disorders involving organs that undergo self-regeneration, including the skin. Genetic reversion may occur through different mechanisms, and in a single individual, the mutation can be repaired in various ways. Here we describe a disseminated pattern of revertant mosaicism observed in 6 patients with Kindler syndrome (KS), a genodermatosis caused by loss of kindlin-1 (encoded by FERMT1) and clinically characterized by patchy skin pigmentation and atrophy. All patients presented duplication mutations (c.456dupA and c.676dupC) in FERMT1, and slipped mispairing in direct nucleotide repeats was identified as the reversion mechanism in all investigated revertant skin spots. The sequence around the mutations demonstrated high propensity to mutations, favoring both microinsertions and microdeletions. Additionally, in some revertant patches, mitotic recombination generated areas with homozygous normal keratinocytes. Restoration of kindlin-1 expression led to clinically and structurally normal skin. Since loss of kindlin-1 severely impairs keratinocyte proliferation, we predict that revertant cells have a selective advantage that allows their clonal expansion and, consequently, the improvement of the skin condition. Find the latest version: https://jci.me/61976/pdf Brief report Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi,1 Yinghong He,1 Anna M.G. Pasmooij,2 Meltem Onder,3 Rudolf Happle,1 Marcel F. -

EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies
Focusing on Personalised Medicine EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies Extended carrier screening is an important tool for prospective parents to help them determine their risk of having a child affected with a heritable disease. In many cases, parents aren’t aware they are carriers and have no family history due to the rarity of some diseases in the general population. What is covered by the screening? Genomics For Life offers a comprehensive Extended Carrier Screening test, providing prospective parents with the information they require when planning their pregnancy. Extended Carrier Screening has been shown to detect carriers who would not have been considered candidates for traditional risk- based screening. With a simple mouth swab collection, we are able to test for over 419 genes associated with inherited diseases, including Fragile X Syndrome, Cystic Fibrosis and Spinal Muscular Atrophy. The assay has been developed in conjunction with clinical molecular geneticists, and includes genes listed in the NIH Genetic Test Registry. For a list of genes and disorders covered, please see the reverse of this brochure. If your gene of interest is not covered on our Extended Carrier Screening panel, please contact our friendly team to assist you in finding a gene test panel that suits your needs. Why have Extended Carrier Screening? Extended Carrier Screening prior to pregnancy enables couples to learn about their reproductive risk and consider a complete range of reproductive options, including whether or not to become pregnant, whether to use advanced reproductive technologies, such as preimplantation genetic diagnosis, or to use donor gametes. -

A Mutation in Lamin A/C Gene Previously Known to Cause Emery
ical C lin as C e f R o l e a p n o r r t u s o J Journal of Clinical Case Reports Chalissery et al., J Clin Case Rep 2016, 6:4 ISSN: 2165-7920 DOI: 10.4172/2165-7920.1000770 Case Report Open Access A Mutation in Lamin A/C Gene Previously Known to Cause Emery- Driefuss Muscular Dystrophy Causing A Phenotype of Limb Girdle Muscular Dystrophy Type 1B Albi J Chalissery1*, Tudor Munteanu1, Yvonne Langan2, Francesca Brett2 and Janice Redmond1 1Department of Neurology, St James’s Hospital, Ireland 2Department of Neurophysiology, St James’s Hospital, Ireland 3Department of Neuropathology, Beaumont Hospital, Dublin, Ireland *Corresponding author: Albi J Chalissery, Department of Neurology, St James’s Hospital, James’s Street, Dublin 8, Ireland, Tel +353 1 410 3000; E-mail: [email protected] Rec date: Feb 19, 2016; Acc date: Apr 13, 2016; Pub date: Apr 18, 2016 Copyright: © 2016 Chalissery AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Mutations in the lamin protein(found in the nuclear envelope) known to cause different allelic disorders including limb girdle muscular dystrophies (LGMD) and Emery-Dreifuss muscular dystrophy (EDMD). LGMDs are a heterogeneous group of disorders with progressive proximal muscle weakness in an autosomal inheritance pattern. LGMD type 1B is a disorder secondary to a mutation in the gene encoding Lamin A/C protein in the nuclear envelope. -

Harlequin Ichthyosis
orphananesthesia Anaesthesia recommendations for patients suffering from Harlequin ichthyosis Disease name: Harlequin ichthyosis ICD 10: Q80.4 Synonyms: Harlequin baby, ichthyosis congenita, Ichthyosis fetalis, keratosis diffusa fetalis, Harlequin fetus, Ichthyosis congenita gravior Disease summary: Harlequin ichthyosis (HI) is an autosomal recessive congenital ichthyosis. HI is an extremely rare and most severe form of ichthyosis. The condition is caused by mutation of the ABCA12 gene resulting in impaired lipid transport in the outermost layer of the skin, the epidermis. During the neontatal period, harlequin ichthyosis manifests phenotypically as dramatic large polygonal plate-like scaling of the skin that cracks and can slough, revealing the underlying diffusely bright red skin. These thick skin plates can pull and distort facial features. The tightness of the skin can also pull on the eyes and mouth resulting in difficulties with closing these structures. The tightness also causes the eyes and the mouth to turn inside out resulting in ectropion and eclabium. Other features include hypoplasia of the fingers, malformation of the ears and nose, and alopecia. Affected neonates often do not survive and mortality is commonly attributed to respiratory failure and/or sepsis. Clinical data obtained from 45 HI patients revealed 25 survivors and 20 deaths with an overall survival rate of only 56%. The ages of survivors ranged from 10 months to 25 years and death usually occurred in the first 3 months. HI infants need to be cared for in a neonatal intensive care unit immediately after birth. Several harlequin neonates have survived. They tend to have severe erythroderma and fine scaling, even with optimal management. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -
Blueprint Genetics Ectodermal Dysplasia Panel
Ectodermal Dysplasia Panel Test code: DE0401 Is a 25 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of ectodermal dysplasia (hidrotic or hypohidrotic) or Ellis-van Creveld syndrome. About Ectodermal Dysplasia Ectodermal Dysplasia (ED) is a group of closely related conditions of which more than 150 different syndromes have been identified. EDs affects the development or function of teeth, hair, nails and sweat glands. ED may present as isolated or as part of a syndromic disease and is commonly subtyped according to sweating ability. The clinical features of the X-linked and autosomal forms of hypohidrotic ectodermal dysplasia (HED) can be indistinguishable and many of the involved genes may lead to phenotypically distinct outcomes depending on number of defective alleles. The most common EDs are hypohidrotic ED and hydrotic ED. X-linked hypohidrotic ectodermal dysplasia (HED) is caused by EDA mutations and explain 75%-95% of familial HED and 50% of sporadic cases. HED is characterized by three cardinal features: hypotrichosis (sparse, slow-growing hair and sparse/missing eyebrows), reduced sweating and hypodontia (absence or small teeth). Reduced sweating poses risk for episodes of hyperthermia. Female carriers may have some degree of hypodontia and mild hypotrichosis. Isolated dental phenotypes have also been described. Mutations in WNT10A have been reported in up to 9% of individuals with HED and in 25% of individuals with HED who do not have defective EDA. Approximately 50% of individuals with heterozygous WNT10A mutation have HED and the most consistent clinical feature is severe oligodontia of permanent teeth. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -
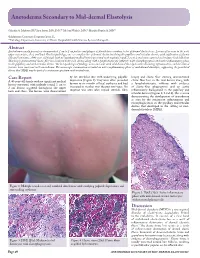
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years.