CDC Recommendations for HPV Vaccine 2-Dose Schedules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Supportive Science Regarding Thimerosal Removal
Summary of Supportive Science Regarding Thimerosal Removal Updated December 2012 www.safeminds.org Science Summary on Mercury in Vaccines (Thimerosal Only) SafeMinds Update – December 2012 Contents ENVIRONMENTAL IMPACT ................................................................................................................................. 4 A PILOT SCALE EVALUATION OF REMOVAL OF MERCURY FROM PHARMACEUTICAL WASTEWATER USING GRANULAR ACTIVATED CARBON (CYR 2002) ................................................................................................................................................................. 4 BIODEGRADATION OF THIOMERSAL CONTAINING EFFLUENTS BY A MERCURY RESISTANT PSEUDOMONAS PUTIDA STRAIN (FORTUNATO 2005) ......................................................................................................................................................................... 4 USE OF ADSORPTION PROCESS TO REMOVE ORGANIC MERCURY THIMEROSAL FROM INDUSTRIAL PROCESS WASTEWATER (VELICU 2007)5 HUMAN & INFANT RESEARCH ............................................................................................................................ 5 IATROGENIC EXPOSURE TO MERCURY AFTER HEPATITIS B VACCINATION IN PRETERM INFANTS (STAJICH 2000) .................................. 5 MERCURY CONCENTRATIONS AND METABOLISM IN INFANTS RECEIVING VACCINES CONTAINING THIMEROSAL: A DESCRIPTIVE STUDY (PICHICHERO 2002) ...................................................................................................................................................... -

Supplemental Information and Guidance for Vaccination Providers Regarding Use of 9-Valent HPV Vaccine
Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV A 9-valent human papillomavirus (HPV) vaccine (9vHPV, Gardasil 9, Merck & Co.) was licensed for use in females and males in December 2014.1,2,3,4 The 9vHPV was the third HPV vaccine licensed in the United States by the Food and Drug Administration (FDA); the other vaccines are bivalent HPV vaccine (2vHPV, Cervarix, GlaxoSmithKline), licensed for use in females, and quadrivalent HPV vaccine (4vHPV, Gardasil, Merck & Co.), licensed for use in females and males.5 In February 2015, the Advisory Committee on Immunization Practices (ACIP) recommended 9vHPV as one of three HPV vaccines that can be used for routine vaccination of females and one of two HPV vaccines for routine vaccination of males.6 After the end of 2016, only 9vHPV will be distributed in the United States. In October 2016, ACIP updated HPV vaccination recommendations regarding dosing schedules.7 CDC now recommends two doses of HPV vaccine (0, 6–12 month schedule) for persons starting the vaccination series before the 15th birthday. Three doses of HPV vaccine (0, 1–2, 6 month schedule) continue to be recommended for persons starting the vaccination series on or after the 15th birthday and for persons with certain immunocompromising conditions. Guidance is needed for persons who started the series with 2vHPV or 4vHPV and may be completing the series with 9vHPV. The information below summarizes some of the recommendations included in ACIP Policy Notes and provides additional guidance.5-7 Information about the vaccines What are some of the similarities and differences between the three HPV vaccines? y Each of the three HPV vaccines is a noninfectious, virus-like particle (VLP) vaccine. -

Pediatric Immunizations: Immunization Current Recommended Chedule and Ecommendations Immunization Schedule
Online Continuing Education for Nurses Linking Learning to Performance INSIDE THIS COURSE PEDIATRIC IMMUNIZATIONS: IMMUNIZATION CURRENT RECOMMENDED SCHEDULE AND RECOMMENDATIONS IMMUNIZATION SCHEDULE ................ 1 ROUTINE IMMUNIZATIONS ............. 2 COMBINATION VACCINES .............. 6 2.27 Contact Hours VACCINATION ADMINISTRATION ......... 7 Written By: Erin Azuse, RN, BSN VACCINATION REACTIONS ................ 8 CONTRAINDICATIONS TO OBJECTIVES RECEIVING VACCINATIONS ............... 9 1. List the current recommended schedule of MISCONCEPTIONS ABOUT VACCINATIONS ................................ 9 immunizations per the CDC and discuss how the list is updated. THE FUTURE FOR PEDIATRIC IMMUNIZATIONS ............................. 10 2. Identify 10 preventable diseases for which REFERENCES ................................ 11 children routinely receive immunizations. CE EXAM...................................... 13 3. Discuss symptoms and complications of these EVALUATION ................................. 16 diseases. 4. Be familiar with combination vaccines and their components. 5. Explain the proper nursing procedure for administering vaccinations. 6. Identify the potential reactions that may occur from immunizations. 7. Describe any contraindications to receiving immunizations. 8. Identify common misconceptions about vaccinations and describe the nurse’s role in changing this. CURRENT RECOMMENDED IMMUNIZATION SCHEDULE The Centers for Disease Control and Prevention (CDC) recommends a schedule of routine vaccinations to protect against -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

Hepatitis B Vaccination Schedule and PVS Guidance for Infants Born to Hepatitis B Positive Women
Hepatitis B Vaccination Schedule and PVS Guidance for Infants Born to Hepatitis B Positive Women Pediatric Hepatitis B Vaccination Schedule and Product Options Following the birth dose of hepatitis B vaccine and HBIG, the infant needs either two doses of monovalent hepatitis B vaccine or three doses of a hepatitis B-containing combination vaccine to complete the hepatitis B vaccine series. In particular, please note: . Pediarix should not be administered before 6 weeks of age. The final dose of hepatitis B vaccine (either the third or fourth dose) should be given on or after 6 months of age. Do not give it before 24 weeks of age. Comvax is not approved for use in infants born to hepatitis B positive women OPTION 1: Monovalent hepatitis B vaccine schedule (Energix or Recombivax) Dose Timing Dose 1 (given with HBIG) Within 12 hours after birth Dose 2 1-2 months of age Dose 3 6 months of age Post-vaccination serology (HBsAg and anti-HBs) 1-2 months after last dose, but not before 9 months of age OPTION 2: Pediarix vaccine schedule Dose Timing Dose of monovalent hep B vaccine (given with HBIG) Within 12 hours after birth Dose 2 6 weeks to 2 months of age (do not administer before 6 weeks of age) Dose 3 4 months of age Dose 4 6 months of age Post-vaccination serology (HBsAg and anti-HBs) 1-2 months after last dose, but not before 9 months of age Post-vaccination Serology (PVS) Results and Recommended Follow-up Serology result Follow-up needed HBsAg negative None. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -
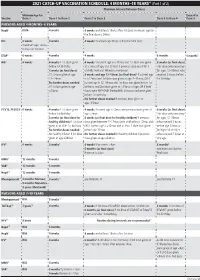
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses Minimum Age for Dose 4 to Vaccine Dose 1 Dose 1 to Dose 2 Dose 2 to Dose 3 Dose 3 to Dose 4 Dose 5 PERSONS AGED 4 MONTHS–6 YEARS HepB1 Birth 4 weeks 8 weeks and at least 16wks after 1st dose; minimum age for the final dose is 24wks RV2 6 weeks 4 weeks 4 weeks maximum age 8mos, 0 days for final dose maximum age 14wks, 6 days for 1st dose DTaP3 6 weeks 4 weeks 4 weeks 6 months 6 months3 Hib4 6 weeks 4 weeks if 1st dose given 4 weeks4 if current age is <12mos and 1st dose was given 8 weeks (as final dose) before 1st birthday at <7mos of age, and at least 1 previous dose was PRP-T This dose only necessary 8 weeks (as final dose) (ActHib, Pentacel, Hiberix) or unknown for ages 12–59mos who if 1st dose given at age 8 weeks and age 12–59mos (as final dose)4 if current age received 3 doses before 12–14mos is <12mos and 1st dose was given at age 7–11mos; OR if 1st birthday No further doses needed current age is 12–59mos and 1st dose was given before 1st if 1st dose given at age birthday, and 2nd dose given at <15mos of age; OR if both ≥15mos doses were PRP-OMP (PedvaxHIB; Comvax) and were given before 1st birthday No further doses needed if previous dose given at age ≥15mos PCV13, PPSV235 6 weeks 4 weeks if 1st dose given 4 weeks if current age <12mos and previous dose given at 8 weeks (as final dose) before 1st birthday age <7mos This dose only necessary 8 weeks (as final dose for 8 weeks (as final dose for healthy children) if previous for ages 12–59mos healthy children) if 1st dose dose given between 7–11mos (wait until at least 12mos old); who received 3 doses given at or after 1st birthday OR if current age is ≥12mos and at least 1 dose was given before age 12mos or No further doses needed before age 12mos. -

Vaccine Myths: Setting the Record Straight
Journal of Family Strengths Volume 18 Issue 1 Critical Issues: Defining and Debunking Misconceptions in Health, Education, Criminal Article 13 Justice, and Social Work/Social Services October 2018 Vaccine Myths: Setting the Record Straight Julie A. Boom Baylor College of Medicine, [email protected] Rachel M. Cunningham Texas Childrens Hospital, [email protected] Lindy U. McGee Baylor College of Medicine, [email protected] Follow this and additional works at: https://digitalcommons.library.tmc.edu/jfs Recommended Citation Boom, Julie A.; Cunningham, Rachel M.; and McGee, Lindy U. (2018) "Vaccine Myths: Setting the Record Straight," Journal of Family Strengths: Vol. 18 : Iss. 1 , Article 13. Available at: https://digitalcommons.library.tmc.edu/jfs/vol18/iss1/13 The Journal of Family Strengths is brought to you for free and open access by CHILDREN AT RISK at DigitalCommons@The Texas Medical Center. It has a "cc by-nc-nd" Creative Commons license" (Attribution Non- Commercial No Derivatives) For more information, please contact [email protected] Boom et al.: Vaccine Myths Introduction Vaccines are one of the most important public health achievements of the 20th century and are responsible for the steep decline in vaccine- preventable diseases (VPDs) in the U.S. The incidence of most VPDs in the U.S. has declined by 90 to 100% (Centers for Disease Control and Prevention [CDC], 1999) (see Table 1). Table 1. Vaccine-preventable diseases: post-vaccine percent decrease in morbidity. Pre-Vaccine Estimated 2016 Reported Percentage Disease Annual Cases* Decrease Morbidity† Smallpox 29,005 0 100% Diphtheria 21,053 0 100% Measles 530,162 85 99.98% Mumps 155,760 6,369 95.91% Pertussis 185,120 17,972 90.29% Polio (paralytic) 16,316 0 100% Rubella 47,734 1 100% Congenital Rubella 151 2 98.68% Syndrome Tetanus 539 34 93.69% †Source: Roush, Murphy, and the Vaccine-Preventable Disease Table Working Group (2007). -

Addressing Parental Vaccine Hesitancy Towards Childhood Vaccines in the United States: a Systematic Literature Review of Communication Interventions and Strategies
Review Addressing Parental Vaccine Hesitancy towards Childhood Vaccines in the United States: A Systematic Literature Review of Communication Interventions and Strategies Olivia Olson 1,*, Corinne Berry 2 and Nirbhay Kumar 1,* 1 Department of Global Health, Milken Institute School of Public Health, The George Washington University, Washington, DC 20052, USA 2 CommunicateHealth, Rockville, MD 20850, USA; [email protected] * Correspondence: [email protected] (O.O.); [email protected] (N.K.) Received: 15 September 2020; Accepted: 4 October 2020; Published: 8 October 2020 Abstract: Parental vaccine hesitancy is becoming an increasingly important public health concern in the United States. In March 2020, an assessment of the latest CDC National Immunization Survey data found that more than one-third of U.S. children between the ages of 19 and 35 months were not following the recommended early childhood immunization schedule. Furthermore, a 2019 national survey found that approximately 1 in 4 parents reported serious concerns towards vaccinating their children. Vaccine hesitancy is now associated with a decrease in vaccine coverage and an increase in vaccine-preventable disease outbreaks and epidemics in the United States. Many studies have focused on understanding and defining the new socio-medical term, vaccine hesitancy; few have attempted to summarize past and current health communication interventions and strategies that have been successful or unsuccessful in tackling this growing phenomenon. This systematic literature review will attempt to aid public health professionals with a catalogue of health communication interventions and strategies to ultimately address and prevent parental vaccine hesitancy in the long term. Out of 1239 search results, a total of 75 articles were included for analysis, ranging from systematic reviews, quantitative surveys, and experimental designs to ethnographic and qualitative studies. -

Hepatitis B Vaccine and Hepatitis B Immune Globulin Administration For
Hepatitis B Vaccine and Hepatitis B Immune Globulin Administration for Infants Maternal Status Infants greater than or equal to 2000 g * Infants less than 2000 g * GIVE single antigen hepatitis B (hepB) vaccine and hepatitis B GIVE single antigen hepB vaccine and HBIG within 12 hours of immune globulin (HBIG) within 12 hours of birth. birth. Do not count the hepB birth dose as the first dose. Since MCIR does not currently assess for infants less than 2,000 grams, COMPLETE hepB vaccine series with single antigen doses at 1-2 please make a note in the medical chart to repeat the infant’s birth and 6 months of age or hepB-containing combination vaccines dose of hepB vaccine at 1 month of age. given at 2, 4, and 6 months of age. (Combination vaccines cannot be given before 6 weeks of age.) Complete the full hepB vaccine series by giving another single- antigen dose at 1 month and additional doses at 2-3 and 6 months of Hepatitis B Surface TEST for hepatitis B surface antibody (anti-HBs) and HBsAg at 9- age or hepB-containing combination vaccines given at 2, 4, and 6 Antigen (HBsAg) 12 months of age (1-2 months after the final dose if the vaccine months of age. (Combination vaccines cannot be given before 6 positive series is delayed). weeks of age.) If infant is anti-HBs-positive, no additional vaccine or testing and needed, infant is protected from the HBV. TEST for anti-HBs and HBsAg at 9-12 months of age (1-2 months If infant is HBsAg and anti-HBs negative, GIVE one after the final dose if the vaccine series is delayed). -

Uproar Over a Little-Known Preservative, Thimerosal, Jostles U.S
Uproar over a little-known preservative, thimerosal, jostles U.S. hepatitis B vaccination policy In 1997, when Frank Pallone, a Democratic congressman from New Jersey, attached a simple amendment to an FDA reauthorization bill, he could not have predicted that it would cause such a commotion two years later. His amendment ran just 133 words. It gave FDA two years to "compile a list of drugs and foods that contain intentionally introduced mercury compounds and … [to] provide a quantitative and qualitative analysis of the mercury compounds in the list…." The bill later evolved into the landmark FDA Modernization Act of 1997 (FDAMA) and was signed into law on November 21, 1997. Pallone’s amendment undoubtedly sprang from his long interest in environmental causes. But he had unwittingly set into motion a chain of events that would, two years later, bring turmoil to the immunization policy world and fears of harm to the nation’s hepatitis B control effort. Thimerosal, old soldier under a cloud At first glance, someone looking for a controversy would not choose thimerosal. It has been used as a vaccine preservative since the 1930s, and, until recently, it has generally been viewed as a safe, reliable, and somewhat drab defender against bacterial and fungal contamination. The compound garners only one short paragraph in the 1249-page Plotkin and Orenstein reference book, Vaccines 3rd Edition (1999). Thimerosal is sometimes added to vaccines during manufacturing as a guarantee against production-related contamination. Its greatest value, however, is in the field, where it acts as a fail-safe against imperfect aseptic handling. -

Top 20 Questions About Vaccination
Top 20 Questions about Vaccination 1. How do vaccines work? Do they work against viruses and bacteria? 2. Why aren’t all vaccines 100% effective? 3. Why are there so many vaccines? 4. Is natural immunity better than vaccine acquired immunity? 5. Why do some vaccines require boosters? 6. My child was invited to a chicken pox party. Would it be better for my child to get the chicken pox this way? Why do we vaccinate against a mild disease like chicken pox? 7. Can you get a disease from the vaccine that’s supposed to prevent it? And why do some vaccines have live pathogens but others have killed pathogens? 8. Can babies’ immune systems handle so many vaccines? 9. Why is there a new flu vaccine every year? 10. What is herd immunity? Is it real? Does it work? 11. Why is allergy to eggs a contraindication to getting some vaccines? 12. Do vaccines cause autism? 13. People say that vaccines are linked to long-term health problems such as multiple sclerosis, diabetes, and autism. Is that true? 14. The vaccine information sheet for my child’s recent vaccination listed lots of potential side effects. Why is vaccination recommended if it can cause all of these side effects? 15. Do we do enough safety testing with vaccines? 16. Do vaccines have aborted fetal tissue? 17. Isn’t it true that better hygiene and nutrition were responsible for decreases in deaths and disease rates, rather than vaccines? 18. Why can’t we eradicate other diseases, as we did with smallpox? 19.