12498.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Supportive Science Regarding Thimerosal Removal
Summary of Supportive Science Regarding Thimerosal Removal Updated December 2012 www.safeminds.org Science Summary on Mercury in Vaccines (Thimerosal Only) SafeMinds Update – December 2012 Contents ENVIRONMENTAL IMPACT ................................................................................................................................. 4 A PILOT SCALE EVALUATION OF REMOVAL OF MERCURY FROM PHARMACEUTICAL WASTEWATER USING GRANULAR ACTIVATED CARBON (CYR 2002) ................................................................................................................................................................. 4 BIODEGRADATION OF THIOMERSAL CONTAINING EFFLUENTS BY A MERCURY RESISTANT PSEUDOMONAS PUTIDA STRAIN (FORTUNATO 2005) ......................................................................................................................................................................... 4 USE OF ADSORPTION PROCESS TO REMOVE ORGANIC MERCURY THIMEROSAL FROM INDUSTRIAL PROCESS WASTEWATER (VELICU 2007)5 HUMAN & INFANT RESEARCH ............................................................................................................................ 5 IATROGENIC EXPOSURE TO MERCURY AFTER HEPATITIS B VACCINATION IN PRETERM INFANTS (STAJICH 2000) .................................. 5 MERCURY CONCENTRATIONS AND METABOLISM IN INFANTS RECEIVING VACCINES CONTAINING THIMEROSAL: A DESCRIPTIVE STUDY (PICHICHERO 2002) ...................................................................................................................................................... -

Supplemental Information and Guidance for Vaccination Providers Regarding Use of 9-Valent HPV Vaccine
Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV A 9-valent human papillomavirus (HPV) vaccine (9vHPV, Gardasil 9, Merck & Co.) was licensed for use in females and males in December 2014.1,2,3,4 The 9vHPV was the third HPV vaccine licensed in the United States by the Food and Drug Administration (FDA); the other vaccines are bivalent HPV vaccine (2vHPV, Cervarix, GlaxoSmithKline), licensed for use in females, and quadrivalent HPV vaccine (4vHPV, Gardasil, Merck & Co.), licensed for use in females and males.5 In February 2015, the Advisory Committee on Immunization Practices (ACIP) recommended 9vHPV as one of three HPV vaccines that can be used for routine vaccination of females and one of two HPV vaccines for routine vaccination of males.6 After the end of 2016, only 9vHPV will be distributed in the United States. In October 2016, ACIP updated HPV vaccination recommendations regarding dosing schedules.7 CDC now recommends two doses of HPV vaccine (0, 6–12 month schedule) for persons starting the vaccination series before the 15th birthday. Three doses of HPV vaccine (0, 1–2, 6 month schedule) continue to be recommended for persons starting the vaccination series on or after the 15th birthday and for persons with certain immunocompromising conditions. Guidance is needed for persons who started the series with 2vHPV or 4vHPV and may be completing the series with 9vHPV. The information below summarizes some of the recommendations included in ACIP Policy Notes and provides additional guidance.5-7 Information about the vaccines What are some of the similarities and differences between the three HPV vaccines? y Each of the three HPV vaccines is a noninfectious, virus-like particle (VLP) vaccine. -

Pediatric Immunizations: Immunization Current Recommended Chedule and Ecommendations Immunization Schedule
Online Continuing Education for Nurses Linking Learning to Performance INSIDE THIS COURSE PEDIATRIC IMMUNIZATIONS: IMMUNIZATION CURRENT RECOMMENDED SCHEDULE AND RECOMMENDATIONS IMMUNIZATION SCHEDULE ................ 1 ROUTINE IMMUNIZATIONS ............. 2 COMBINATION VACCINES .............. 6 2.27 Contact Hours VACCINATION ADMINISTRATION ......... 7 Written By: Erin Azuse, RN, BSN VACCINATION REACTIONS ................ 8 CONTRAINDICATIONS TO OBJECTIVES RECEIVING VACCINATIONS ............... 9 1. List the current recommended schedule of MISCONCEPTIONS ABOUT VACCINATIONS ................................ 9 immunizations per the CDC and discuss how the list is updated. THE FUTURE FOR PEDIATRIC IMMUNIZATIONS ............................. 10 2. Identify 10 preventable diseases for which REFERENCES ................................ 11 children routinely receive immunizations. CE EXAM...................................... 13 3. Discuss symptoms and complications of these EVALUATION ................................. 16 diseases. 4. Be familiar with combination vaccines and their components. 5. Explain the proper nursing procedure for administering vaccinations. 6. Identify the potential reactions that may occur from immunizations. 7. Describe any contraindications to receiving immunizations. 8. Identify common misconceptions about vaccinations and describe the nurse’s role in changing this. CURRENT RECOMMENDED IMMUNIZATION SCHEDULE The Centers for Disease Control and Prevention (CDC) recommends a schedule of routine vaccinations to protect against -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

Hepatitis B Vaccination Schedule and PVS Guidance for Infants Born to Hepatitis B Positive Women
Hepatitis B Vaccination Schedule and PVS Guidance for Infants Born to Hepatitis B Positive Women Pediatric Hepatitis B Vaccination Schedule and Product Options Following the birth dose of hepatitis B vaccine and HBIG, the infant needs either two doses of monovalent hepatitis B vaccine or three doses of a hepatitis B-containing combination vaccine to complete the hepatitis B vaccine series. In particular, please note: . Pediarix should not be administered before 6 weeks of age. The final dose of hepatitis B vaccine (either the third or fourth dose) should be given on or after 6 months of age. Do not give it before 24 weeks of age. Comvax is not approved for use in infants born to hepatitis B positive women OPTION 1: Monovalent hepatitis B vaccine schedule (Energix or Recombivax) Dose Timing Dose 1 (given with HBIG) Within 12 hours after birth Dose 2 1-2 months of age Dose 3 6 months of age Post-vaccination serology (HBsAg and anti-HBs) 1-2 months after last dose, but not before 9 months of age OPTION 2: Pediarix vaccine schedule Dose Timing Dose of monovalent hep B vaccine (given with HBIG) Within 12 hours after birth Dose 2 6 weeks to 2 months of age (do not administer before 6 weeks of age) Dose 3 4 months of age Dose 4 6 months of age Post-vaccination serology (HBsAg and anti-HBs) 1-2 months after last dose, but not before 9 months of age Post-vaccination Serology (PVS) Results and Recommended Follow-up Serology result Follow-up needed HBsAg negative None. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Development of Piagetian Object Permanence in a Grey Parrot (Psittacus Erithacus)
WellBeing International WBI Studies Repository 3-1997 Development of Piagetian Object Permanence in a Grey Parrot (Psittacus erithacus) Irene M. Pepperberg University of Arizona Mark R. Willner University of Arizona Lauren B. Gravitz Barnard College Follow this and additional works at: https://www.wellbeingintlstudiesrepository.org/acwp_asie Part of the Animal Studies Commons, Comparative Psychology Commons, and the Other Animal Sciences Commons Recommended Citation Pepperberg, I. M., Willner, M. R., & Gravitz, L. B. (1997). Development of Piagetian object permanence in grey parrot (Psittacus erithacus). Journal of Comparative Psychology, 111(1), 63. This material is brought to you for free and open access by WellBeing International. It has been accepted for inclusion by an authorized administrator of the WBI Studies Repository. For more information, please contact [email protected]. Development of Piagetian Object Permanence in a Grey Parrot (Psittacus erithacus) Irene M. Pepperberg*, Mark R. Willner*, and Lauren B. Gravitz† * University of Arizona † Barnard College ABSTRACT The authors evaluated the ontogenetic performance of a grey parrot (Psittacus erithacus) on object permanence tasks designed for human infants. Testing began when the bird was 8 weeks old, prior to fledging and weaning. Because adult grey parrots understand complex invisible displacements (I. M. Pepperberg & F. A. Kozak, 1986), the authors continued weekly testing until the current subject completed all of I. C. Uzgiris and J. Hunt's (1975) Scale 1 tasks. Stage 6 object permanence with respect to these tasks emerged at 22 weeks, after the bird had fledged but before it was completely weaned. Although the parrot progressed more rapidly overall than other species that have been tested ontogenetically, the subject similarly exhibited a behavioral plateau part way through the study. -

Chapter 4 a Cultural Approach to Child Development
Child Development A Cultural Approach Chapter 4 Infancy Copyright © 2017, 2013 Pearson Education, Inc. All Rights Reserved Learning Objectives (1 of 5) 4.1 Describe how the infant’s body changes in the first year, and explain the two basic principles of physical growth. 4.2 Identify the different parts of the brain and describe how the brain changes in the first few years of life. 4.3 Describe how infant sleep changes in the course of the first year and evaluate risk factors for SIDS, including the research evidence regarding cosleeping. Copyright © 2017, 2013 Pearson Education, Inc. All Rights Reserved Learning Objectives (2 of 5) 4.4 Describe how infants’ nutritional needs change during the first year of life and identify the reasons for and consequences of malnutrition in infancy. 4.5 List the major causes and preventive methods of infant mortality and describe some cultural approaches to protecting infants. 4.6 Describe the major changes during infancy in gross and fine motor development. 4.7 Describe how infants’ sensory abilities develop in the first year. Copyright © 2017, 2013 Pearson Education, Inc. All Rights Reserved Learning Objectives (3 of 5) 4.8 Describe the first four sensorimotor substages of Piaget’s theory. 4.9 Describe how the elements of the information- processing model of cognitive functioning change in infancy. 4.10 Describe the major scales used in measuring infant development and explain how habituation assessments are used to predict later intelligence. 4.11 Evaluate the claim that educational media enhance infants’ cognitive development. Copyright © 2017, 2013 Pearson Education, Inc. -

Snapshots* Developmental Milestones
The Division of Developmental Pediatrics, Department of Pediatrics, Faculty of Medicine and Dentistry, University of Alberta SNAPSHOTS* DEVELOPMENTAL MILESTONES Mnemonic Gotta Find Strong Coffee Soon‡ G = Gotta F = Find S = Strong C = Coffee S = Soon Age Gross Motor Fine Motor Speech / Language Cognitive / Problem Solving Social / Emotional Newborn Primitive reflexes – step, place, Primitive reflexes – grasp Primitive reflexes – root, suck Visual focal length ~10” Bonding (parent child) Moro, Babinski, ATNR Alerts to sound Fix & follow slow horizontal arc Prefers Self-regulation/soothing Flexor posture Startles to loud sounds contrast, colours, face Variable cries Prefers high pitched voice 2 mos Head steady when held Hands open half of time Turns to voice Prefers usual caregiver Attachment (child parent) Head up 45o prone Bats at objects Cooing Attends to moderate novelty Social smile Follows past midline 4 mos Sits with support Palmar grasp Laugh, razz, "ga", squeal Anticipates routines Turn-taking conversations Head up 90o prone, arms out Reaches and obtains items Purposeful sensory exploration of objects Explores parent's face Rolls front back Brings objects to midline (eyes, hands, mouth) 6 mos Postural reflexes Raking grasp Babble (nonspecific) Stranger anxiety Expresses emotions: happy, sad, Sits tripod Transfers hand to hand Looks for dropped or partially hidden mad Rolls both ways object Memory lasts ~24 hrs 9 mos Gets from all 4s sitting Inferior pincer grasp "Mama", "dada" (specific) Object permanence Separation anxiety -

Executive Function in Infants and Toddlers Born Low Birth Weight and Preterm
Executive Function in Infants and Toddlers born Low Birth Weight and Preterm Patricia M Blasco, PhD, Sybille Guy, PhD, & Serra Acar, PhD The Research Institute Objectives • Participants will understand retrospective research on young children born LBW and later school outcomes. • Participants will learn preliminary findings from this and other studies by the researchers. • Participants will engage in active planning for state activities to insure children born LBW are followed at birth and monitored for development. Executive Function Refers to a group of neurocognitive processes in the brain that direct, connect, and organize information that is manifested in planned behavior. She’s the CEO of her brain Early Childhood and EF • Components follow their own developmental trajectory • Growth spurts in the last half of the first year and then from 3 to 6 years of age (Diamond, 2006) Why are these so important in Early Childhood? • Inability to plan and organize actions, maintain attention to tasks, and recall past experience to apply to new learning experiences lead to: • Learning disabilities (LD) as well as problems with Attention-deficit/hyperactivity disorder (ADHD)(Lyon & Fletcher, 2001). Neurocognitive Processes • Self Regulation • Cognitive Flexibility • Goal Selection • Inhibition • Planning and • Working Memory Organization Self-Regulation Self-regulation functions are developing from the first years of life on throughout a person’s entire lifetime. Inhibit Ability to control behavior and impulses Redirect Activity Stop, Think & -

Early Intervention in Pediatric Occupational Therapy
Chapter 1 Early Intervention in Pediatric Occupational Therapy Serkan Pekçetin and Ayla Günal Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.68316 Abstract Early intervention is services for infants and toddlers who have developmental defi‐ ciency or considered high risk due to the environmental or biologic factors. The aim of the early intervention is increasing the physical, cognitive and emotional capacities of infants/toddlers with protecting them from the environmental or biological risk factors. Early intervention should start as soon as possible for obtaining the best results for the child and family. First 3 years of life are critical period of the child development because neurologic development still continues. Infants and toddlers are providing physical, cognitive, sensory and social development with different experiences and various‐ sen sory stimuli from the environment in this period. Occupational therapists evaluate and implement interventions to activity, environment, infant/toddlers and their families for minimizing the developmental risks. For these reasons, occupational therapists are considered important members of early intervention team. Keywords: early intervention, occupational therapy, sensory motor performance, play therapy, cognitive, feeding disorders, social development 1. Introduction 1.1. High risk infant This term is using for the infant who has increasing risk for disability, but the exact disability is not actualized yet. The risk factors of infants can be divided into two main subheadings. The first subheading is biological risk factors. These are: intracranial hemorrhage, diabetic retinopathy, sepsis, necrotizing enterocolitis, apnea, asphyxia, intraventricular hemorrhage and the brachial plexus injury. The second subheading is environmental risk factors. -
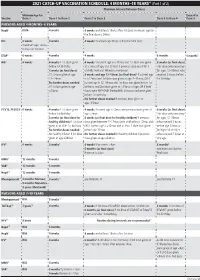
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses Minimum Age for Dose 4 to Vaccine Dose 1 Dose 1 to Dose 2 Dose 2 to Dose 3 Dose 3 to Dose 4 Dose 5 PERSONS AGED 4 MONTHS–6 YEARS HepB1 Birth 4 weeks 8 weeks and at least 16wks after 1st dose; minimum age for the final dose is 24wks RV2 6 weeks 4 weeks 4 weeks maximum age 8mos, 0 days for final dose maximum age 14wks, 6 days for 1st dose DTaP3 6 weeks 4 weeks 4 weeks 6 months 6 months3 Hib4 6 weeks 4 weeks if 1st dose given 4 weeks4 if current age is <12mos and 1st dose was given 8 weeks (as final dose) before 1st birthday at <7mos of age, and at least 1 previous dose was PRP-T This dose only necessary 8 weeks (as final dose) (ActHib, Pentacel, Hiberix) or unknown for ages 12–59mos who if 1st dose given at age 8 weeks and age 12–59mos (as final dose)4 if current age received 3 doses before 12–14mos is <12mos and 1st dose was given at age 7–11mos; OR if 1st birthday No further doses needed current age is 12–59mos and 1st dose was given before 1st if 1st dose given at age birthday, and 2nd dose given at <15mos of age; OR if both ≥15mos doses were PRP-OMP (PedvaxHIB; Comvax) and were given before 1st birthday No further doses needed if previous dose given at age ≥15mos PCV13, PPSV235 6 weeks 4 weeks if 1st dose given 4 weeks if current age <12mos and previous dose given at 8 weeks (as final dose) before 1st birthday age <7mos This dose only necessary 8 weeks (as final dose for 8 weeks (as final dose for healthy children) if previous for ages 12–59mos healthy children) if 1st dose dose given between 7–11mos (wait until at least 12mos old); who received 3 doses given at or after 1st birthday OR if current age is ≥12mos and at least 1 dose was given before age 12mos or No further doses needed before age 12mos.