1960 Studies of the Chromosomes of North
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title Lorem Ipsum Dolor Sit Amet, Consectetur Adipiscing Elit
Volume 26: 102–108 METAMORPHOSIS www.metamorphosis.org.za ISSN 1018–6490 (PRINT) LEPIDOPTERISTS’ SOCIETY OF AFRICA ISSN 2307–5031 (ONLINE) Classification of the Afrotropical butterflies to generic level Published online: 25 December 2015 Mark C. Williams 183 van der Merwe Street, Rietondale, Pretoria, South Africa. E-mail: [email protected] Copyright © Lepidopterists’ Society of Africa Abstract: This paper applies the findings of phylogenetic studies on butterflies (Papilionoidea) in order to present an up to date classification of the Afrotropical butterflies to genus level. The classification for Afrotropical butterflies is placed within a worldwide context to subtribal level. Taxa that still require interrogation are highlighted. Hopefully this classification will provide a stable context for researchers working on Afrotropical butterflies. Key words: Lepidoptera, Papilionoidea, Afrotropical butterflies, classification. Citation: Williams, M.C. (2015). Classification of the Afrotropical butterflies to generic level. Metamorphosis 26: 102–108. INTRODUCTION Suborder Glossata Fabricius, 1775 (6 infraorders) Infraorder Heteroneura Tillyard, 1918 (34 Natural classifications of biological organisms, based superfamilies) on robust phylogenetic hypotheses, are needed before Clade Obtectomera Minet, 1986 (12 superfamilies) meaningful studies can be conducted in regard to their Superfamily Papilionoidea Latreille, 1802 (7 evolution, biogeography, ecology and conservation. families) Classifications, dating from the time of Linnaeus in the Family Papilionidae Latreille, 1802 (32 genera, 570 mid seventeen hundreds, were based on morphology species) for nearly two hundred and fifty years. Classifications Family Hedylidae Guenée, 1858 (1 genus, 36 species) based on phylogenies derived from an interrogation of Family Hesperiidae Latreille, 1809 (570 genera, 4113 the genome of individual organisms began in the late species) 20th century. -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

Perceptual Range, Targeting Ability, and Visual Habitat Detection by Greater Fritillary Butterfliesspeyeria Cybele (Lepidoptera: Nymphalidae) and Speyeria Atlantis
Journal of Insect Science, (2019) 19(4): 1; 1–10 doi: 10.1093/jisesa/iez060 Research Perceptual Range, Targeting Ability, and Visual Habitat Detection by Greater Fritillary ButterfliesSpeyeria cybele (Lepidoptera: Nymphalidae) and Speyeria atlantis Zachary G. MacDonald,1,4, John H. Acorn,1, Jian Zhang,2,3, and Scott E. Nielsen1, Downloaded from https://academic.oup.com/jinsectscience/article-abstract/19/4/1/5525229 by guest on 18 July 2019 1Department of Renewable Resources, University of Alberta, Edmonton, Alberta, 751 General Services Building, Edmonton, Alberta, T6G 2H1, Canada, 2Zhejiang Tiantong Forest Ecosystem National Observation and Research Station, School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, P.R. China, 3Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, P.R. China, and 4Corresponding author, e-mail: [email protected] Subject Editor: Phyllis Weintraub Received 4 February 2019; Editorial decision 26 May 2019 Abstract Butterflies are widely invoked as model organisms in studies of metapopulation and dispersal processes. Integral to such investigations are understandings of perceptual range; the maximum distance at which organisms are able to detect patches of suitable habitat. To infer perceptual range, researchers have released butterflies at varying distances from habitat patches and observed their subsequent flight behaviors. It is often assumed that butterflies rely on visual senses for habitat detection; however, this assumption has not been explicitly investigated. Here, we assess the extent and sensory determinants of perceptual range for the great spangled fritillary (Speyeria cybele (Fabricius, 1775)) and Atlantis fritillary (Speyeria atlantis (W.H. Edwards, 1862)). This was achieved by experimentally releasing butterflies over open water at various distances from a lake island, representing an isolated habitat patch in a dichotomous habitat-matrix landscape. -

A Rearing Method for Argynnis (Speyeria) Diana
Hindawi Publishing Corporation Psyche Volume 2011, Article ID 940280, 6 pages doi:10.1155/2011/940280 Research Article ARearingMethodforArgynnis (Speyeria) diana (Lepidoptera: Nymphalidae) That Avoids Larval Diapause Carrie N. Wells, Lindsey Edwards, Russell Hawkins, Lindsey Smith, and David Tonkyn Department of Biological Sciences, Clemson University, 132 Long Hall, Clemson, SC 29634, USA Correspondence should be addressed to Carrie N. Wells, [email protected] Received 25 May 2011; Accepted 4 August 2011 Academic Editor: Russell Jurenka Copyright © 2011 Carrie N. Wells et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We describe a rearing protocol that allowed us to raise the threatened butterfly, Argynnis diana (Nymphalidae), while bypassing the first instar overwintering diapause. We compared the survival of offspring reared under this protocol from field-collected A. diana females from North Carolina, Georgia, and Tennessee. Larvae were reared in the lab on three phylogenetically distinct species of Southern Appalachian violets (Viola sororia, V. pubescens,andV. pedata). We assessed larval survival in A. diana to the last instar, pupation, and adulthood. Males reared in captivity emerged significantly earlier than females. An ANOVA revealed no evidence of host plant preference by A. diana toward three native violet species. We suggest that restoration of A. diana habitat which promotes a wide array of larval and adult host plants, is urgently needed to conserve this imperiled species into the future. 1. Introduction larvae in cold storage blocks and storing them under con- trolled refrigerated conditions for the duration of their The Diana fritillary, Argynnis (Speyeria) diana (Cramer overwintering period [10]. -

Journal of the Lepidopterists' Society
J OURNAL OF T HE L EPIDOPTERISTS’ S OCIETY Volume 62 2008 Number 2 Journal of the Lepidopterists’ Society 61(2), 2007, 61–66 COMPARATIVE STUDIES ON THE IMMATURE STAGES AND DEVELOPMENTAL BIOLOGY OF FIVE ARGYNNIS SPP. (SUBGENUS SPEYERIA) (NYMPHALIDAE) FROM WASHINGTON DAVID G. JAMES Department of Entomology, Washington State University, Irrigated Agriculture Research and Extension Center, 24105 North Bunn Road, Prosser, Washington 99350; email: [email protected] ABSTRACT. Comparative illustrations and notes on morphology and biology are provided on the immature stages of five Arg- ynnis spp. (A. cybele leto, A. coronis simaetha, A. zerene picta, A. egleis mcdunnoughi, A. hydaspe rhodope) found in the Pacific Northwest. High quality images allowed separation of the five species in most of their immature stages. Sixth instars of all species possessed a fleshy, eversible osmeterium-like gland located ventrally between the head and first thoracic segment. Dormant first in- star larvae of all species exposed to summer-like conditions (25 ± 0.5º C and continuous illumination), 2.0–2.5 months after hatch- ing, did not feed and died within 6–9 days, indicating the larvae were in diapause. Overwintering of first instars for ~ 80 days in dark- ness at 5 ± 0.5º C, 75 ± 5% r.h. resulted in minimal mortality. Subsequent exposure to summer-like conditions (25 ± 0.5º C and continuous illumination) resulted in breaking of dormancy and commencement of feeding in all species within 2–5 days. Durations of individual instars and complete post-larval feeding development durations were similar for A. coronis, A. zerene, A. egleis and A. -

1996 No. 4 December
TROPICAL LEPIDOPTERA NEWS December 1996 No.4 LEPIDOPTERORUM CATALOGUS (New Series) The new world catalog of Lepidoptera renews the series title The new series (as edited by J. B. Heppner) began already in first begun in 1911. The original catalog series was published by 1989 with publication of the catalog of Noctuidae, by R. Poole. W. Junk Publishers of Berlin, Germany (later The Hague, E. J. Brill Publishers, of Leiden, Netherlands, published this first Netherlands), continuing until 1939 when the incomplete series fascicle in 3 volumes, covering already about a third of all known was deactivated due to World War II. The original series Lepidoptera. Since ATL took over the series, several families completed a large number of families between 1911 and 1939, have been readied for publication. Already this month, Fascicle totalling about 3 shelf-feet of text. Most Microlepidoptera, 48, on Epermeniidae, was published (authored by R. Gaedike, of however, were not covered, as also several macro families like the Deutsches Entomologisches Institut, Eberswalde, Germany). Noctuidae, and several families are incomplete (e.g., Geometridae In 1997, several other smaller families are expected, including and Pyralidae). Even for what was treated, the older catalogs are Acanthopteroctetidae (Davis), Acrolepiidae (Gaedike), Cecidosi now greatly out of date, due to the description of many new dae (Davis), Cercophanidae (Becker), Glyphipterigidae (Heppner), species and many changes in nomenclature over the last 5 to 8 Neotheoridae (Kristensen), Ochsenheimeriidae (Davis), Opostegi decades. dae (Davis), and Oxytenidae (Becker). Much of the publication The new series resembles the old series in some ways but it schedule depends on the cooperation of various specialists who will also have features not found in the old work. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Lepidoptera für 1903. Bearbeitet von Dr. Robert Lucas in Rixdorf bei Berlin. A. Publikationen (Autoren alphabetisch) mit Referaten. Adkin, Robert. Pyrameis cardui, Plusia gamma and Nemophila noc- tuella. The Entomologist, vol. 36. p. 274—276. Agassiz, G. Etüde sur la coloration des ailes des papillons. Lausanne, H. Vallotton u. Toso. 8 °. 31 p. von Aigner-Abafi, A. (1). Variabilität zweier Lepidopterenarten. Verhandlgn. zool.-bot. Ges. Wien, 53. Bd. p. 162—165. I. Argynnis Paphia L. ; IL Larentia bilineata L. — (2). Protoparce convolvuli. Entom. Zeitschr. Guben. 17. Jahrg. p. 22. — (3). Über Mimikry. Gaea. 39. Jhg. p. 166—170, 233—237. — (4). A mimicryröl. Rov. Lapok, vol. X, p. 28—34, 45—53 — (5). A Mimicry. Allat. Kozl. 1902, p. 117—126. — (6). (Über Mimikry). Allgem. Zeitschr. f. Entom. 7. Bd. (Schluß p. 405—409). Über Falterarten, welche auch gesondert von ihrer Umgebung, in ruhendem Zustande eine eigentümliche, das Auge täuschende Form annehmen (Lasiocampa quercifolia [dürres Blatt], Phalera bucephala [zerbrochenes Ästchen], Calocampa exoleta [Stück morschen Holzes]. — [Stabheuschrecke, Acanthoderus]. Raupen, die Meister der Mimikry sind. Nachahmung anderer Tiere. Die Mimik ist in vielen Fällen zwecklos. — Die wenn auch recht geistreichen Mimikry-Theorien sind doch vielleicht nur ein müßiges Spiel der Phantasie. Aitken u. Comber, E. A list of the butterflies of the Konkau. Journ. Bombay Soc. vol. XV. p. 42—55, Suppl. p. 356. Albisson, J. Notes biologiques pour servir ä l'histoire naturelle du Charaxes jasius. Bull. Soc. Etud. Sc. nat. Nimes. T. 30. p. 77—82. Annandale u. Robinson. Siehe unter S w i n h o e. -
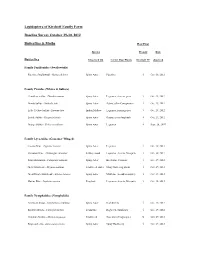
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Ronciers Genève
Ronciers Ronciers Pruno-Rubion Profil Identité Surface 57 ha (0.2% de la surface cantonale) Equivalence : Code du milieu : 516 Sec Humide Guide des milieux naturels de Suisse : 5.3.3, 5.3.4 Humidité EUNIS : F3.111 CORINE : 31.811 Acide Alcalin Acidité Protection : – Richesse Faible Elevée REG : agricole en nutriments Grossier Fin Granulométrie 1 2 3 4 5 Naturel Artificiel Naturalité ■ 1 2 3 4 5 Description Valeur biologique Les ronciers constituent le plus souvent des fourrés Les ronciers jouent un rôle important dans la protection des denses. Ils se développent sur des terrains très riches sols de l’espace rural en limitant l’érosion superficielle par en nutriments* et bien ensoleillés. Très envahissants, ils leur ancrage racinaire2. Ils sont également importants pour tendent à former des massifs monospécifiques impéné- la biodiversité*, car ils forment des structures favorables à la trables de 2 à 3 m de haut, avec un optimum de crois- faune (insectes, petits et grands mammifères, oiseaux) en sance sur les terrains dénudés laissés à l’abandon1. Ils offrant des sites d’alimentation, de reproduction, ainsi que se rencontrent également dans les clairières, le long des de nombreux abris2. C’est particulièrement vrai pour les nom- lisières forestières, au contact des haies ou sur les talus breux papillons et abeilles sauvages auxquels les ronciers ferroviaires2. offrent gîte et nourriture4. La carte cantonale des milieux regroupe à l’échelle du 1 : 5’000e S’ils bénéficient d’une certaine largeur et d’un linéaire suffi- les deux variantes suivantes : sant, les ronciers peuvent fonctionner comme des corridors • les groupements de ronces indigènes* (Pruno-Rubion : biologiques2 et s’intégrer au paysage bocager, au même titre Groupement à Rubus fruticosus) dominés par la ronce que les cordons d’espèces ligneuses ou que les formations commune (Rubus fruticosus aggr.)3 ; buissonnantes. -

Butterflies in the Verde Valley
Butterflies in the Verde Valley 1 2 17 1. Mormon Fritillary, Speyeria mormonia 2. Empress Leilia, Asterocampa leilia 3. Fiery Skipper, Hylephila phyleus 4. Greenish-Blue Lycaenid, Plebejus saepiolus 16 4 18 Female on top, male below 3 5. Pipevine Swallowtail, Battus philenor 6. American Snout Butterfly, Libytheana carinenta 7. Cloudless Sulpher, Phoebis sennae 14 15 Female with patterned wing, male solid 5 8. Two-tailed Swallowtail, Papilio multicaudata 9. Variegated Fritillary, Euptoieta claudia 13 12 10. Hoary Comma, Polygonia gracilis 6 11. California Sister, Adelpha bredowii Eurema nicippe 8 12. Sleepy Orange Sulphur, 11 Two males 10 13. Alfalfa Sulfpur, Colias euretheme Two males, one female (pale) 14. Painted Lady, Vanessa cardui 15. Pine White, Neophasia menapia 9 7 16. Viceroy, Limenitus archippus 17. Queen, Danaus gilippus 18. Black Swallowtail, Papilio polyxenes Butterflies Butterflies are an amazing group of insects, and their delicate structure, flight, and colors, brighten our day. The butterflies in this display represent 6 related families of Lepidoptera (scaled wings) which we know as butterflies. All of these occur in northern Arizona. The best place to look for butterflies is often in moist creek beds and in areas where flowers are blooming. The mouth is composed of two long flexible straws that are connected. When they feed/drink they unroll the mouth parts (called a proboscis) and suck in nectar or other liquids. Although the shapes and colors differs, their basic structure is similar. All have four wings, six legs, an abdomen, thorax, head, and antenna. Butterflies have complete metamorphosis, with four separate stages: (1)egg, (2)larva, (3)chrysalis, and (4)adult. -

Poleward Shifts in Geographical Ranges of Butterfly Species Associated with Regional Warming
letters to nature between 270 and 4,000 ms after target onset) and to ignore changes in the distractor. Failure to respond within a reaction-time window, responding to a change in the distractor or deviating the gaze (monitored with a scleral search Poleward shifts in coil) by more than 1Њ from the fixation point caused the trial to be aborted without reward. The change in the target and distractors was selected so as to geographical ranges of be challenging for the animal. In experiments 1 and 2 the animal correctly completed, on average, 79% of the trials, broke fixation in 11%, might have butterfly species associated responded to the distractor stimulus in 6% and responded too early or not at all in 5% of the trials. In Experiment 3 the corresponding values are 78, 13%, 8% with regional warming and 2%. In none of the three experiments was there a difference between the Camille Parmesan*†, Nils Ryrholm‡, Constantı´ Stefanescu§, performances for the two possible targets. Differences between average eye Jane K. Hillk, Chris D. Thomas¶, Henri Descimon#, positions during trials where one or the other stimulus was the target were Brian Huntleyk, Lauri Kaila!, Jaakko Kullberg!, very small, with only an average shift of 0.02Њ in the direction of the shift of Toomas Tammaru**, W. John Tennent††, position between the stimuli. Only correctly completed trials were considered. Jeremy A. Thomas‡‡ & Martin Warren§§ Firing rates were determined by computing the average neuronal response * National Center for Ecological Analysis and Synthesis, 735 State Street, across trials for 1,000 ms starting 200 ms after the beginning of the target Suite 300, Santa Barbara, California 93101, USA stimulus movement.