Post-Zygotic Reproductive Isolation Among Populations of Iris Atropurpurea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scanned Document
•••••OCTOBER · 19 7 4- Number 14 THE SPECIES IRIS STUDY GROUP OF THE AMERICAN IRIS SOCIETY Jean Witt, of Seattle, is the Director of the AIS Species Seed Exchange. he also is an expert at doing ink-line botanic illustrations. Her seed exchange list for 1974 will be exten ive - but it will NOT offer eeds of the pecies which she has drawn for this cover of SIGNA. Seeds of Iris afghanica, of tpe Regelia Section, are not yet available - because Iris afghanico is a newly discovered and newly described species. More details are on page 367. THE SPECIES IRIS STUDY GROUP of_ TH E AMERICAN IRIS SOCIETY OFFICERS OF THE SOCIETY Chairman- - - - - - - Roy Davidson- - - 911 Western Avenue,,_ Number 200 Seattle, Washington !:18104 phone 206-746- 2156 Secretary-Treasurer - - - Homer Metcalf - - Montana State Universi~i College of Agriculture BoLeman Montana 597 5 phone 46 6-586-5624 Librarian - - - - - - Jerry Flintoff- 5608 North 18th Street Tacoma, Washi:1gton 98406 Seed Exchange Director Jean Witt - 16516 25th, N.E. Seattle, Washington 98155 Species Robins Director- Lorena Reid 17225 McKenzie Highwa'i, Route 2 Springfield, Oregon 97477 Editor of SIGNA - - - Bill Gunther 740 Crest Road Del Ma.c, California 92014 phone , 14-755- 2798 Editor of Study Manual Roy Davidson- - 911 Western Avenue,,_ Number 200 Seattle, Washington !:18104 • • • • • • • • • • • SIGNA - - - Number 14 OCTOBER 1974 TABLE OF CONTENTS Cover--lris afghanica · Jean Witt - · · · 353 Notes on SIGNA 13 · - Roy Davidson - - · 355 It is a Gift! - - - - - Bill Gunther · · · 356 The Genus Iris: a review - - - - - - - P.J. Chittenden - - 357 Spuria Species as Garden Plants - E. -

These De Doctorat De L'universite Paris-Saclay
NNT : 2016SACLS250 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de doctorat (Biologie) Par Mlle Nour Abdel Samad Titre de la thèse (CARACTERISATION GENETIQUE DU GENRE IRIS EVOLUANT DANS LA MEDITERRANEE ORIENTALE) Thèse présentée et soutenue à « Beyrouth », le « 21/09/2016 » : Composition du Jury : M., Tohmé, Georges CNRS (Liban) Président Mme, Garnatje, Teresa Institut Botànic de Barcelona (Espagne) Rapporteur M., Bacchetta, Gianluigi Università degli Studi di Cagliari (Italie) Rapporteur Mme, Nadot, Sophie Université Paris-Sud (France) Examinateur Mlle, El Chamy, Laure Université Saint-Joseph (Liban) Examinateur Mme, Siljak-Yakovlev, Sonja Université Paris-Sud (France) Directeur de thèse Mme, Bou Dagher-Kharrat, Magda Université Saint-Joseph (Liban) Co-directeur de thèse UNIVERSITE SAINT-JOSEPH FACULTE DES SCIENCES THESE DE DOCTORAT DISCIPLINE : Sciences de la vie SPÉCIALITÉ : Biologie de la conservation Sujet de la thèse : Caractérisation génétique du genre Iris évoluant dans la Méditerranée Orientale. Présentée par : Nour ABDEL SAMAD Pour obtenir le grade de DOCTEUR ÈS SCIENCES Soutenue le 21/09/2016 Devant le jury composé de : Dr. Georges TOHME Président Dr. Teresa GARNATJE Rapporteur Dr. Gianluigi BACCHETTA Rapporteur Dr. Sophie NADOT Examinateur Dr. Laure EL CHAMY Examinateur Dr. Sonja SILJAK-YAKOVLEV Directeur de thèse Dr. Magda BOU DAGHER KHARRAT Directeur de thèse Titre : Caractérisation Génétique du Genre Iris évoluant dans la Méditerranée Orientale. Mots clés : Iris, Oncocyclus, région Est-Méditerranéenne, relations phylogénétiques, status taxonomique. Résumé : Le genre Iris appartient à la famille des L’approche scientifique est basée sur de nombreux Iridacées, il comprend plus de 280 espèces distribuées outils moléculaires et génétiques tels que : l’analyse de à travers l’hémisphère Nord. -
Positive and Negative Impacts of Non-Native Bee Species Around the World
Supplementary Materials: Positive and Negative Impacts of Non-Native Bee Species around the World Laura Russo Table S1. Selected references of potential negative impacts of Apis or Bombus species. Bold, underlined, and shaded text refers to citations with an empirical component while unbolded text refers to papers that refer to impacts only from a hypothetical standpoint. Light grey shading indicates species for which neither positive nor negative impacts have been recorded. “But see” refers to manuscripts that show evidence or describe the opposite of the effect and is capitalized when only contradictory studies could be found. Note that Apis mellifera scutellata (the “Africanized” honeybee), is treated separately given the abundance of research specifically studying that subspecies. Altering Non-native Nesting Floral Pathoens/ Invasive Introgres Decrease Pollination Species Sites Resources Parasites Weeds sion Plant Fitness Webs Apis cerana [1] [2] [1–3] [4] Apis dorsata Apis florea [5] [5] [37,45] But see [8–19] but [27–35] but [36–38] [39–43] [38,46,47] Apis mellifera [9,23–26] [4] [6,7] see [6,20–22] see [6] but see [44] [48,49] but see [50] Apis mellifera [51] but see [55–57] scutellata [52–54] Bombus [58,59] hortorum Bombus But see But see [60] [61] hypnorum [60] Bombus [62] [62,63] [26,64–66] [62] impatiens Bombus lucorum Bombus [28,58,59,6 [39] but see [67,68] [69,70] [36,39] ruderatus 9,71,72] [73] Bombus [59] subterraneous [67,70,74,75, [29,58,72,9 Bombus [25,26,70,7 [38,39,68,81,97,98 [4,76,88, [47,76,49,86,97 [74–76] 77–84] but 1–95] but terrestris 6,87–90] ] 99,100] ,101–103] see [85,86] see [96] Insects 2016, 7, 69; doi:10.3390/insects7040069 www.mdpi.com/journal/insects Insects 2016, 7, 69 S2 of S8 Table S2. -

Pollinator Limitation on Reproductive Success in Iris Tuberosa
Research Article Pollinator limitation on reproductive success in Iris tuberosa Giuseppe Pellegrino* Department of Biology, Ecology and Earth Science, University of Calabria, I-87036 Rende, Italy Received: 22 September 2014; Accepted: 8 December 2014; Published: 19 December 2014 Associate Editor: James F. Cahill Citation: Pellegrino G. 2015. Pollinator limitation on reproductive success in Iris tuberosa. AoB PLANTS 7: plu089; doi:10.1093/aobpla/plu089 Abstract. Variation in plant and floral size can have conflicting effects on pollination and fruit production in flower- ing plants. This research examines the contributions of plant height, flower size and pollinator visitation to reproduct- ive success in four populations of Iris tuberosa. The plants were pollinated exclusively by hymenopteran species, primarily during sunny days. Pollination supplementation increased the proportion of flowers that matured into fruit, with 95 % fruit set for hand-pollinated compared with 74.15 % for naturally pollinated flowers. The pollinator visitation rate and the proportion of fruit produced were not significantly different between tall and short plants or between small and large flowers. Furthermore, the increase in plant size and floral display did not increase the frequency of pollinator visitations and so did not increase the fruit set. Thus, despite the widespread effects of flower- ing plant size on pollinator attraction and plant reproduction in other species, these effects are lacking in I. tuberosa. This study quantifies the role of pollinators in the reproductive success of I. tuberosa. Pollinators visited tall/short plants and large/small flowers in equal proportion, suggesting that plant and floral display size do not affect pollinator attraction and reproductive success in I. -

Fine-Grained Flower Classification
Fine-Grained Flower Classification Ravid Cohen Prof Daphna Weinshall (Computer Science) Prof Avi Shmida (Botanic) The Hebrew University of Jerusalem 1. Introduction Recently models for fine-grained flower-based classification tasks, in particular models that use features from pre-trained convolutional neural network as an image representation, show outstanding results, with more than 95% mean class accuracy on the Oxford Flower Database [4][6][7]. It would seem that these emerging tools may be able to address the more realistic and challenging problems posed by the varying appearance of flowers in the wild, including ‘super-fine-grained’ classification tasks. In accordance, we collected a new and even more fine-grained database of Israeli wild flowers with varying levels of similarity between categories. We then followed the classification paradigm described in Razavian et al [1] to classify both the Oxford 102 flower database and the new one. Using the most recent representations based on deep learning networks, the results on the Oxford dataset are excellent, with 94.9% mean class accuracy. When training the same classifier with our database it achieved only 79.3%, an evidence for the higher challenge the Israel Flower Database poses. The Israel Flower Database is also unique in being hierarchical (genus and species), while being labeled with accurate scientific botanical names. 2. Databases Oxford Flowers 102 (OF 102) database [3] Oxford Flowers 102 consists of 8,189 images from 102 categories. Each category contains 40-258 images of common wild flowers in the UK. Most of the categories are genera rather than species and they are labeled using their common English name. -

Iris Section Oncocyclus) and Identification of Flower Development Genes
bioRxiv preprint doi: https://doi.org/10.1101/680363; this version posted June 23, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. De novo Transcriptome Characterization of Royal Iris (Iris section Oncocyclus) and Identification of Flower Development Genes Bar-Lev Yamit1, Senden Esther1, Pasmanik-Chor Metsada2 and Sapir Yuval1 1 The Botanical Garden, School of Plant Sciences and Food Security, G.S. Wise Faculty of Life Science, Tel Aviv University, Israel 2 Bioinformatics Unit, G.S. Wise Faculty of Life Science, Tel Aviv University, Tel Aviv 69978, Israel Bar-Lev Yamit [email protected], 972-3-6407354, Author for correspondence Senden Esther [email protected] Pasmanik-Chor Metsada [email protected] Sapir Yuval [email protected] Acknowledgements We thank the Technion Genome Center for technical assistance and the Bioinformatics Core Facility at Ben-Gurion University for their assistance in the bioinformatics analysis. We thank N. Kane for the Perl script for mining SSRs from transcriptome sequences. This research was funded by the Israel Science Foundation (grant No. 336/16). 1 bioRxiv preprint doi: https://doi.org/10.1101/680363; this version posted June 23, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

De Novo Transcriptome Characterization of Iris Atropurpurea (The Royal Iris, Iris Section Oncocyclus) and Phylogenetic Analysis
bioRxiv preprint doi: https://doi.org/10.1101/680363; this version posted September 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. De novo Transcriptome Characterization of Iris atropurpurea (the Royal Iris, Iris section Oncocyclus) and Phylogenetic Analysis of MADS-box and R2R3- MYB Gene Families Bar-Lev Yamit1, Senden Esther1, Pasmanik-Chor Metsada2, and Sapir Yuval1 1 The Botanical Garden, School of Plant Sciences and Food Security, G.S. Wise Faculty of Life Science, Tel Aviv University, Israel 2 Bioinformatics Unit, G.S. Wise Faculty of Life Science, Tel Aviv University, Tel Aviv 69978, Israel Bar-Lev Yamit [email protected], 972-3-6407354, Author for correspondence Senden Esther [email protected] Pasmanik-Chor Metsada [email protected] Sapir Yuval [email protected] Acknowledgments We thank the Technion Genome Center for technical assistance and the Bioinformatics Core Facility at Ben-Gurion University for their assistance in the bioinformatics analysis. We thank N. Kane for the Perl script for mining SSRs from transcriptome sequences. This research was funded by the Israel Science Foundation to YS (grant No. 336/16). 1 bioRxiv preprint doi: https://doi.org/10.1101/680363; this version posted September 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

N. S. Volume LVIII ANNALI DI BOTANICA 2000 at This Meeting The
n. s. Volume LVIII ANNALI DI BOTANICA 2000 A PRELIMINARY LIST OF RARE OR ENDANGERED IRIDACEAE SPECIES At this meeting the importance of focussing on rare and endangered Iridaceae species in each country became evident. A complete check list of the conservation status of rare and endangered Irid a cea e species is not yet estabilished. Here we present (Appendix) the list of the World Conservation Monitoring Centre (WCMC)* for rare and endangered Iridaceae, according to the IUCN categories. Some problems occur in listing rare and endangered species. For example, although in Israel legislation has protected all Iris species since 1964, so that we have now a complete list, this is not the case in other countries and for other genera. Furthermore, unsolved taxonomic problems confused the issue in some species and the exact distribution of others is still unknown. Here, we want to stimulate colleagues working on Irid a c e a e to send new information to the monitoring centre to improve the current red list. In Italy, we have species of bearded irises which are endangered because of animal and/or human influence. For example, in some areas of Italy, wild boar populations have been promoted to favour hunting, with resulting damage to Iris populations which have been rapidly reduced to a few individuals, as I. relicta Colas, on Monte delle Fate (Southern Lazio), because the boars dig up Iris rhizomes. In other areas, the intensive construction of houses has endangered some populations, for example, in the case of/, setina Colas. (Latina), a bearded Iris that flowers in February. -

Pollination of Oncocyclus Irises (Iris: Iridaceae) by Night-Sheltering Male Bees
Research Paper 417 Pollination of Oncocyclus irises (Iris: Iridaceae) by Night-Sheltering Male Bees Y. Sapir1,3, A. Shmida1, and G. Ne’eman2 1 Department of Evolution, Systematics and Ecology, The Hebrew University of Jerusalem, Givat Ram, Jerusalem, 91904, Israel 2 Dept. of Biology, University of Haifa-Oranim, Tivon, 36006, Israel 3 Present address: Dept. of Biology, Indiana University, Bloomington, IN, 47405, USA Received: January 24, 2005; Accepted: March 10, 2005 Abstract: Irises in the section Oncocyclus (Siems.) Baker (Iris: Iri- iting flowers proved to be their efficient pollinators (Kevan and daceae) grow throughout the Middle East and have large and Baker, 1983).Among the diverse groups of flower-visiting in- dark-coloured flowers but no nectar reward available to flower sects, bees are the best adapted and the most important group visitors. Consequently, no reward-collecting pollinators have of pollinators (Kevan and Baker, 1983; Proctor et al., 1996; been observed visiting the flowers during daytime. The only vis- O’Toole and Raw, 1999), and solitary bees consitute the vast itors are solitarymale bees ( Eucera spp.: Apidae) that enter the majority of bee species (Michener, 2000). flowers at dusk and staythere overnight. Here we describe the mating system of Oncocyclus irises, and the role of night-shelter- Bees are attracted to flowers mainly for the reward offered by ing male bees in their pollination system. Pollen viability in I. the flowers; this reward is mainly nectar and pollen, but other haynei on Mt. Gilboa was veryhigh (> 90%) throughout all floral types of rewards exist (Proctor et al., 1996). Bees use flowers life stages. -

Unlocking the Karyological and Cytogenetic Diversity of Iris from Lebanon: Oncocyclus Section Shows a Distinctive Profile and Re
RESEARCH ARTICLE Unlocking the Karyological and Cytogenetic Diversity of Iris from Lebanon: Oncocyclus Section Shows a Distinctive Profile and Relative Stasis during Its Continental Radiation Nour Abdel Samad1,2, Magda Bou Dagher-Kharrat1*, Oriane Hidalgo3, Rana El Zein1, a11111 Bouchra Douaihy1¤, Sonja Siljak-Yakovlev2 1 Faculté des Sciences, Département Sciences de la Vie et de la Terre, Laboratoire Caractérisation Génomique des Plantes, Campus Sciences et Technologies, Université Saint-Joseph, Mar Roukos Mkalles, Lebanon, 2 Ecologie, Systématique, Evolution, UMR 8079 Univ. Paris-Sud, AgroParisTech, Université Paris-Saclay, Bat. 360, 91405 Orsay, France, 3 Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, United Kingdom ¤ Current address: Faculté des Sciences, Département de Biologie, Université Libanaise, Tripoli, Lebanon OPEN ACCESS * [email protected] Citation: Abdel Samad N, Bou Dagher-Kharrat M, Hidalgo O, El Zein R, Douaihy B, Siljak-Yakovlev S (2016) Unlocking the Karyological and Cytogenetic Diversity of Iris from Lebanon: Oncocyclus Section Abstract Shows a Distinctive Profile and Relative Stasis during Despite being an important target of conservation concern and horticultural interest, Leba- Its Continental Radiation. PLoS ONE 11(8): e0160816. doi:10.1371/journal.pone.0160816 nese irises yet have a confusing taxonomic history and species’ delimitation is often consid- ered problematic, more especially among royal irises (Iris section Oncocyclus). Indeed, Editor: Lorenzo Peruzzi, Università di Pisa, ITALY these irises of exceptionally large and spectacular flowers have radiated across Caucasus Received: March 21, 2016 and eastern Mediterranean giving rise to a number of strict endemic taxa, many of them Accepted: July 26, 2016 being considered under threat. Whilst efforts have mostly focused on clarifying the evolu- Published: August 15, 2016 tionary relationships in the group based on morphological and molecular data, karyological Copyright: © 2016 Abdel Samad et al. -
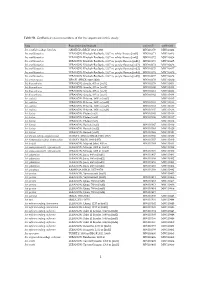
Table S1. Genbank Accession Numbers of the Iris Sequenced in This Study
Table S1. GenBank accession numbers of the Iris sequenced in this study. Taxa Population [individual] trnL-trnF matK-trnK Iris acutiloba subsp. lineolata ARMENIA (RBGK 2012-1109) MW110370 MW110422 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, white flowers [ind1] MW110371 MW110423 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, white flowers [ind2] MW110372 MW110424 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind1] MW110373 MW110425 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind2] MW110374 MW110426 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind3] MW110375 MW110427 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind4] MW110376 MW110428 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind5] MW110377 MW110429 Iris atropurpurea ISRAEL (RBGK 1998-2808) MW110378 MW110430 Iris bismarkiana LEBANON: Sarada, 435 m [ind1] MW110379 MW110431 Iris bismarkiana LEBANON: Sarada, 435 m [ind2] MW110380 MW110432 Iris bismarkiana LEBANON: Sarada, 435 m [ind3] MW110381 MW110433 Iris bismarkiana LEBANON: Sarada, 435 m [ind4] MW110382 MW110434 Iris cedretii LEBANON: Bcharre, 1900 m [ind1] MW110435 Iris cedretii LEBANON: Bcharre, 1900 m [ind2] MW110383 MW110436 Iris cedretii LEBANON: Bcharre, 1900 m [ind3] MW110384 MW110437 Iris cedretii LEBANON: Bcharre, 1900 m [ind4] MW110385 MW110438 Iris histrio LEBANON: Ehden [ind1] MW110365 MW110416 Iris histrio LEBANON: Ehden [ind2] MW110366 MW110417 Iris histrio LEBANON: Ehden [ind3] MW110418 Iris histrio LEBANON: Barouk [ind1] MW110367 MW110419 Iris histrio LEBANON: Barouk [ind2] MW110368 MW110420 Iris histrio LEBANON: Barouk [ind3] MW110369 MW110421 Iris iberica subsp. elegantissima TURKEY: 2200 m (RBGK 1999-4347) MW110386 MW110439 Iris kirkwoodiae subsp. kirkwoodiae TURKEY (RBGK 1994-2407) MW110387 MW110440 Iris lortetii LEBANON: Mays el Jabal, 640 m MW110388 MW110441 Iris mesopotamica (I. -

NOM NOM Plantlist Lieu De Récolte Origine Hemerocallis Esculenta Koidzumi Hemerocallis Esculenta Koidz
NOM NOM Plantlist Lieu de récolte Origine Hemerocallis esculenta Koidzumi Hemerocallis esculenta Koidz. Xanthorrhoeaceae Iles Sakkhalines J.B. St. Petersbourg (Rs) Hemerocallis flava M Hotta. Hemerocallis lilioasphodelus L. Liliaceae Japon Hemerocallis middendorfii. Hemerocallis middendorffii Trautv. & C.A.Mey. Liliaceae Russie Suisse Hemerocallis middendorfii. Hemerocallis middendorffii Trautv. & C.A.Mey. Liliaceae Lyon Hemerocallis minor Hemerocallis minor Mill. Xanthorrhoeaceae Mt Chamar Daban (Rs) Mojmir Pavleka (Cz) Hemerocallis minor Hemerocallis minor Mill. Xanthorrhoeaceae Altaï Krai, 200 m (Rs) J.B. Osnabrûck (Ge) Iris acutiloba CA Mey. Iris acutiloba C.A.Mey. Iridaceae Iris albertii Regel. Iris albertii Regel. Iridaceae Iris albertii Regel. Iris albertii Regel. Iridaceae Ruffier Iris aphylla L. Iris aphylla L. Iridaceae Iris aphylla L. Iris aphylla L. Iridaceae Iris aphylla. Iris aphylla L. Iridaceae Iris aphylla. Iris aphylla L. Iridaceae Allemagne Iris arenaria W et K. Iris arenaria Waldst. & Kit. Iridaceae Iris atropurpurea Backer. Iris atropurpurea Backer. Iridaceae Iris balcana Janka. Iris reichenbachii Heuff. Iridaceae Iris barbata nana. Iris 'Barbata Nana' Iridaceae Lepage Iris bloudowi Ledeb. Iris bloudowi Ledeb. Iridaceae Kazakhstan Ruffier Iris bloudowi Ledeb. Iris bloudowi Ledeb. Iridaceae Sibérie Chitelet Iris bloudowii Ledeb. Iris bloudowii Ledeb. Iridaceae Iris bracteata S Watson. Iris bracteata S Watson. Iridaceae Iris brevicaulis Iris brevicaulis Raf. Iridaceae Iris bulleyana Iris bulleyana Dykes Iridaceae Cult. E. Lauroz Iris bulleyana Iris bulleyana Dykes Iridaceae Zheduo Shan, Sechuan (Sn) V. Holubec (Cz) Iris bulleyana Dykes Iris bulleyana Dykes Iridaceae Yunnan (Sn) J.B. Nancy (Ga) Iris caroliniana S Wats. Iris virginica L.. Iridaceae Iris caucasica Hoffmann. Iris caucasica Hoffmann. Iridaceae Iris caucasica. Iris caucasica Hoffmann. Iridaceae Iris chamaeiris Bertol.