Carbon Storage and DNA Adsorption in Allophanic Soils and Paleosols: Preliminary Findings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PORTLIGHT� PORTLAND YACHT CLUB MONTHLY June 2014 Volume 64, Number 5
PORTLIGHT� PORTLAND YACHT CLUB MONTHLY June 2014 Volume 64, Number 5 Tom Kelly and his crew during the 2014 Swiftsure www.portlandyc.com | 503-285-1922 | 1241 NE Marine Drive, Portland, Oregon 97211 THE COMMODORE’S COLUMN by Commodore Chris Dorn, S/V Ruffian Susan and I had a great time on Opening Day. With a theme like a lot of us who are mostly out there just for the “Making Memories,” what better yacht to make our flagship than fun. Don’t be surprised at the occasional water Chuck Kellogg’s classic yacht, Phantom, and who better to race us balloon or squirt gun competition and I am around the course than Flag Captain (and sometimes racecar driver) always pleasantly surprised at the number of M/V folks, with roots Harry Braunstein. Shirley was on board as were Terry and Lee in sailing, who participate as crew. Johnson. June is also a month for three mini cruises. These are non-hosted How about S/V Camelot and S/V Galatea leading our fleet through gatherings at specific moorage locations – you just show up, dock the water flying Old Glory and the PYC Burgee? I thought the rep- your boat and let the fun begin. resentative PYC Fleet put on quite a show – Bo Knab would have As June marks the middle of the year you will be hearing more been proud. I want to also thank Captain MacGregor for his role about the many projects that our Committees are continuing to as Fleet Captain, the Former Commodore’s and First Mates, our work on, or about to undertake. -

Memoirs of Hydrography
MEMOIRS 07 HYDROGRAPHY INCLUDING Brief Biographies of the Principal Officers who have Served in H.M. NAVAL SURVEYING SERVICE BETWEEN THE YEARS 1750 and 1885 COMPILED BY COMMANDER L. S. DAWSON, R.N. I 1s t tw o PARTS. P a r t II.—1830 t o 1885. EASTBOURNE: HENRY W. KEAY, THE “ IMPERIAL LIBRARY.” iI i / PREF A CE. N the compilation of Part II. of the Memoirs of Hydrography, the endeavour has been to give the services of the many excellent surveying I officers of the late Indian Navy, equal prominence with those of the Royal Navy. Except in the geographical abridgment, under the heading of “ Progress of Martne Surveys” attached to the Memoirs of the various Hydrographers, the personal services of officers still on the Active List, and employed in the surveying service of the Royal Navy, have not been alluded to ; thereby the lines of official etiquette will not have been over-stepped. L. S. D. January , 1885. CONTENTS OF PART II ♦ CHAPTER I. Beaufort, Progress 1829 to 1854, Fitzroy, Belcher, Graves, Raper, Blackwood, Barrai, Arlett, Frazer, Owen Stanley, J. L. Stokes, Sulivan, Berard, Collinson, Lloyd, Otter, Kellett, La Place, Schubert, Haines,' Nolloth, Brock, Spratt, C. G. Robinson, Sheringham, Williams, Becher, Bate, Church, Powell, E. J. Bedford, Elwon, Ethersey, Carless, G. A. Bedford, James Wood, Wolfe, Balleny, Wilkes, W. Allen, Maury, Miles, Mooney, R. B. Beechey, P. Shortland, Yule, Lord, Burdwood, Dayman, Drury, Barrow, Christopher, John Wood, Harding, Kortright, Johnson, Du Petit Thouars, Lawrance, Klint, W. Smyth, Dunsterville, Cox, F. W. L. Thomas, Biddlecombe, Gordon, Bird Allen, Curtis, Edye, F. -

Annals Section4 Yachts.Pdf
CHAPTER 4 Early Yachts IN THE R.V.Y.C. FROM 1903 TO ABOUT 1933 The following list of the first sail yachts in the Club cannot be said to be complete, nevertheless it provides a record of the better known vessels and was compiled from newspaper files of The Province, News-Advertiser, The World and The Sun during the first three decades of the Club activities. Vancouver newspapers gave very complete coverage of sailing events in that period when yacht racing commanded wide public interest. ABEGWEIT—32 ft. aux. Columbia River centerboard cruising sloop built at Steveston in 1912 for H. C. Shaw, who joined the Club in 1911. ADANAC-18 ft. sloop designed and built by Horace Stone in 1910. ADDIE—27 ft. open catboat sloop built in 1902 for Bert Austin at Vancouver Shipyard by William Watt, the first yacht constructed at the yard. Addie was in the original R.V.Y.C. fleet. ADELPIII—44 ft. schooner designed by E. B. Schock for Thicke brothers. Built 1912, sailed by the Thicke brothers till 1919 when sold to Bert Austin, who sold it in 1922 to Seattle. AILSA 1-28.5 ft. D class aux. yawl, Mower design. Built 1907 by Bob Granger, originally named Ta-Meri. Subsequent owners included Ron Maitland, Tom Ramsay, Alan Leckie, Bill Ball and N. S. McDonald. AILSA II—22.5 ft. D class aux. yawl built 1911 by Bob Granger. Owners included J. H. Willard and Joe Wilkinson. ALEXANDRA-45 ft. sloop designed for R.V.Y.C. syndicate by William Fyfe of Fairlie, Scotland and built 1907 by Wm. -
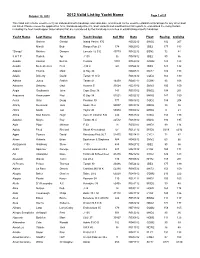
Valid List by Yacht Name Page 1 of 25
October 19, 2012 2012 Valid List by Yacht Name Page 1 of 25 This Valid List is to be used to verify an individual boat's handicap, and valid date, and should not be used to establish a handicaps for any other boat not listed. Please review the appilication form, handicap adjustments, boat variants and modified boat list reports to understand the many factors including the fleet handicapper observations that are considered by the handicap committee in establishing a boat's handicap Yacht Name Last Name First Name Yacht Design Sail Nbr Date Fleet Racing Cruising Gartner Gerald Island Packet 370 R052212 BWS2 192 207 Minelli Bob Ranger Fun 23 174 N062012 JBE2 177 183 "Sloopy" Melcher Dwayne Lacoste 42 S E 40779 R042212 BSN2 72 84 5 H T P Rudich Api J 105 96 R081812 JBE2 90 96 Acadia Keenan Burt H. Custom 1001 R062912 GOM2 123 123 Acadia Biebesheimer Fred J 34 C 69 R052412 JBE2 123 132 Adagio Thuma Mark O Day 30 N040512 MAT2 186 198 Adajio Doherty David Tartan 31 S D R061612 COD2 165 180 Adhara Jones Patrick Tartan 41 14459 R040212 GOM2 93 108 Advance Delaney Ged Avance 33 33524 R021312 SMV2 150 159 Aegis Gaythwaite John Cape Dory 36 141 R051012 BWS2 198 201 Aequoreal Rasmussen Paul O Day 34 51521 R032212 MRN2 147 159 Aerial Gray Doug Pearson 30 777 N061612 COD2 189 204 Affinity Desmond Jack Swan 48-2 50007 R042312 MRN2 33 36 Africa Smith Jud Taylor 45 50974 R030812 MHD2 9 21 Aftica Mac Kenzie Hugh Irwin 31 Citation S D 234 R061512 COD2 183 198 Agadou Mayne Roy Tartan 34 C 22512 R061812 MAN2 180 195 Agila Piper Michael E 33 18 R050912 MHD2 -

NEWPORT BEACH Wooden Boat Festival
NEWPORT BEACH Wooden Boat Festival 2017 NEWPORT BEACH WOODEN BOAT FESTIVAL 1 GET IN ON A $45B CRE MARKETPLACE. TOP BROKERS ARE. Ten-X Commercial is the CRE $10.6MM PURCHASE PRICE 5,607 ASSET PAGE VIEWS marketplace that precision- matches your property to global buyers and unknown buyers. Sixty 109,856 SF percent come from out of state with 36 REGISTERED BUYERS close rates 2X faster than the norm. MULTI-TENANT RETAIL 9 BIDDERS Ten-X.com/commercial | 1-888-770-7332 TenX RE, Inc./Ten-X, a Licensed Real Estate Broker. CA BRE 01994943. For all other state licensing details visit: https://www.ten-x.com/company/legal/licensing/ 1 Mauchly, Irvine, CA 92618 800-499-6199. THE ORIGINAL SOCIAL NETWORKING SITE From its waterfront pools to the expansive fitness center, Balboa Bay Club is the premier members-only gathering place for seekers of luxury and activity. RELAX YOUR OWN WAY 1221 West Coast Highway, Newport Beach, CA 92663 • BalboaBayClub.com • 949.630.4120 We are very pleased to welcome you to the Newport Beach Wooden Boat Festival, a Balboa Yacht Club event. is Festival honors these wooden-boat works of art and salutes their owners who with great devotion, pride and love, maintain these boats in excellent condition so that we can share them with you today. What makes this event so unique is that every boat comes with a story. In some cases these boats are older than us and have lived either a dour or an incredible and abundant life traveling the world’s waterways and oceans with titans of industry or government on their decks. -

Antigua Classic Yacht Regatta See Story on Page 13 TIM WRIGHT / JUNE 2008 CARIBBEAN COMPASS PAGE 2 JUNE 2008 CARIBBEAN COMPASS PAGE 3
C A R I B B E A N On-line C MPASS JUNEJUNE 2008 NO.NO. 153153 TheThe Caribbean’sCaribbean’s Monthly Look atat SeaSea & ShoreShore Antigua Classic Yacht Regatta See story on page 13 TIM WRIGHT / WWW.PHOTOACTION.COM JUNE 2008 CARIBBEAN COMPASS PAGE 2 JUNE 2008 CARIBBEAN COMPASS PAGE 3 ama Puerto Rico St. Croix St. Lucia St. Martin St. Vincent Trinidad and Tobago Antigua Barbados Bequia British Virgin Islands Curacao Croix Puerto Rico St. Panama DominicaGrenada h Virgin Islands Curacao Dominica Pan Grenada Virgin Islands Curacao h St. Lucia St. Martin St. Vincent Trinidad and Tobago Tobago and Trinidad Vincent Martin St. St. Lucia St. t Trinidad and Tobago Antigua Barbados Bequia Britis Tobago and Trinidad t Antigua Barbados Bequia British Virgin Islands Curacao Curacao Islands Virgin British Bequia Barbados Antigua Dominica Grenada Panama Puerto Rico St. Croix Croix St. Rico Puerto Panama Grenada Dominica St. Lucia St. Martin St. Vincen St. Martin St. Lucia St. CALENDAR JUNE 9 Queen’s Birthday (UK). Public holiday in Anguilla 14 – 15 Harris Paints Regatta, Barbados. www.barbadosyachtclub.com 15 Fathers’ Day. Public holiday in Puerto Rico 18 FULL MOON The Caribbean’s Monthly Look at Sea & Shore 19 Labour Day. Public holiday in Trinidad 19 – 22 Scotiabank Opti Regatta, St. Thomas, USVI. www.styc.net www.caribbeancompass.com 20 – 24 La ExpoNáutica Anzoátegui (boat show), Lecherías, Venezuela. www.enoriente.com/expomorro JUNE 2008 • NUMBER 153 21 Summer Solstice 21 Financial Services Challenge Race, BVI. Royal BVI Yacht Club (RBVIYC), tel (284) 494-3286, [email protected], www.rbviyc.net Hurricane 21 International Music Day: Music and Mariners Festival. -

J Ljjf PAGES 7 to 12
: i SPORTS TV MOVIES ICOUNTX CORRESPONDENCE jLXASSIFLEI) 20AREE1S COMICS TWELVE PAGES TWELVE PAGES SECTION TWO SECTION TWO PAGES 7 TO 12 J Ljjf PAGES 7 TO 12 DAILY EAST OREOONIAN, PENDLETON, OREGON, THURSDAY EVENING, JULY 15, 1920. "AMERICA'S CUP"- - -- ITS WINNERS FACTS ABOUT BOATS II AND CHALLENGERS SINCE 1851 IN RACE FOR CUP Use the Phones, Use Phones, CRAFT TUNED FOR CUP Grocery 526 the Vf Defender . Challenger v Challenger' Owner Grocery 526 1M America 44 British sloops , IlIXJIilTI'. .Other Dept's 73 QtArv 170 Mato ' Cambria James Ashbury The IK'fcnilcr : SEHVICE Other Dept's 73 JK71 Columbia l.lvonla James Ashbury RACE OFF SANDY HOOK Owner Syndicate of Xew MEN'S 17 Madeleine Countess of York Yachting enthunlu.su. STORE ' Duffarln ftoyal Canadiun Yacht Cluh 181 Mischief Atalanta Kay f Qulnta Yacht Club 1'llot Rear Commodore Oeo. J8S Puritan Genesta Nocturia, New York Vacht flub. Mayflower Galatea Two Giant Gulls, Resolute and When built 1914, New York. 1887 4-- Weight Lens than 100 tons. Volunteer Thistle J IV, i 189J Vigilant Valkyrie ir Lord Dunraven Shamrock Vie Today for KIIAMKOCK IV 1896 Defender Valkyrie III Ixird Dunraven Crown Which Will Make One Tll CIlHlk'lllwr 1889 Columbia Shamrock sir Thomas Upton Owner Kir Thomas Tipton, 1901 Columbia Shamrock II Sir Thomas Upton Queen of Sea. veteran Irish sportsman. 190 J Reliance Bhamrock Rlr Upton Pilot Capt. William V. Burton, irr Thomas Hoeing 1920 ttesolute Bhamrock IW Sir Thomas LI pi on NEW YORK, July 11!. fir. P.I Yacht .Association of : ITp to dale the defender has never been beaten by the challenger. -

Naval Accidents 1945-1988, Neptune Papers No. 3
-- Neptune Papers -- Neptune Paper No. 3: Naval Accidents 1945 - 1988 by William M. Arkin and Joshua Handler Greenpeace/Institute for Policy Studies Washington, D.C. June 1989 Neptune Paper No. 3: Naval Accidents 1945-1988 Table of Contents Introduction ................................................................................................................................... 1 Overview ........................................................................................................................................ 2 Nuclear Weapons Accidents......................................................................................................... 3 Nuclear Reactor Accidents ........................................................................................................... 7 Submarine Accidents .................................................................................................................... 9 Dangers of Routine Naval Operations....................................................................................... 12 Chronology of Naval Accidents: 1945 - 1988........................................................................... 16 Appendix A: Sources and Acknowledgements........................................................................ 73 Appendix B: U.S. Ship Type Abbreviations ............................................................................ 76 Table 1: Number of Ships by Type Involved in Accidents, 1945 - 1988................................ 78 Table 2: Naval Accidents by Type -

To the Turn of the Century
Chapter 3 TO THE TURN OF THE CENTURY 0n September 2, 1880, the following notice appeared in the Geelong Advertiser:- "A meeting of all interested in forming a Sailing Club will be held at the Victoria Hotel, Friday evening at 8 o'clock." This meeting, convened by Mr. Arthur Speed, took place and the Geelong Sailing Club was constituted. In order to provide members, who did not own yachts of their own, with boats to sail, arrangements were made with Mr. Clement Blunt by which the Sailing Club was provided with three yachts for the use of members and a club room was rented at his (Blunt's), boatshed on the Eastern Beach. A set of rules for the new club were formulated as follows:- SAILING CLUB RULES 1. SAILING CLUB. The club shall be called The Geelong Sailing Club. 2. COLOURS. The club flag shall be red with a white cross. 3. ANNUAL SUBSCRIPTION. The annual subscription shall be three guineas per annum payable as follows. One half on the 1stof October and the balance on the 1st February. In the event of any member's removal from Geelong before the 1st of February in any year, the second half of his subscription shall not become payable. Any new arrival in the town, elected a member after the 1st of January shall pay a subscription of two guineas instead of three. 4. SEASON. The season shall commence on the 1stof September and end on the 30th April. 5. MEMBERS' PRIVILEGES. Any member shall be entitled to take a boat out on any day except Sunday, subject to Rule No. -

Wooldridge Steamboat List
Wooldridge Steamboat List Vessel Name Type Year [--] Ashley 1838 [--] McLean (J.L. McLean) 1854 A. Cabbano Side Wheel Steamboat 1860 A. Fusiler (A. Fuselier) 1851 A. Fusiler (A. Fusilier) 1839 A. Gates Side Wheel Towboat 1896 A. Giles Towboat 1872 A. McDonald Stern Towboat 1871 A. Saltzman Stern Wheel Steamboat 1889 A.B. Chambers Side Wheel Steamboat 1855 A.B. Shaw 1847 A.C. Bird Stern Wheel Steamboat 1875 A.C. Goddin 1856 A.D. Allen Stern Wheel Steamboat 1901 A.D. Hine (Ad Hine) 1860 A.D. Owens Stern Wheel Steamboat 1896 A.D. Taylor Side Wheel Steamboat A.G. Brown Side Wheel Steamboat 1860 A.G. Henry Stern Wheel Steamboat 1880 A.G. Mason Stern Wheel Steamboat 1855 A.G. Ross Stern Wheel Steamboat 1858 A.G. Wagoner Snagboat 1882 A.H. Seviers 1843 A.H. Seviers (A.H. Sevier) 1860 A.J. Sweeny (A.J. Sweeney) Stern Wheel Steamboat 1863 A.J. Baker Towboat 1864 A.J. White Side Wheel Steamboat 1871 A.J. Whitney Stern Towboat 1880 A.L. Crawford Stern Wheel Steamboat 1884 A.L. Davis 1853 Tuesday, June 28, 2005 Page 1 of 220 Vessel Name Type Year A.L. Gregorie (A.L. Gregoire) Ferry 1853 A.L. Mason Stern Wheel Steamboat 1890 A.L. Milburn 1856 A.L. Norton Stern Wheel Steamboat 1886 A.L. Shotwell Side Wheel Steamboat 1852 A.M. Jarrett Stern Wheel Steamboat 1881 A.M. Phillips Side Wheel Steamboat 1835 A.M. Scott Screw Tunnel 1906 A.N. Johnson Side Wheel Steamboat 1842 A.O. Tyler Side Wheel Steamboat 1857 A.R. -

Civil War Manuscripts
CIVIL WAR MANUSCRIPTS CIVIL WAR MANUSCRIPTS MANUSCRIPT READING ROW '•'" -"•••-' -'- J+l. MANUSCRIPT READING ROOM CIVIL WAR MANUSCRIPTS A Guide to Collections in the Manuscript Division of the Library of Congress Compiled by John R. Sellers LIBRARY OF CONGRESS WASHINGTON 1986 Cover: Ulysses S. Grant Title page: Benjamin F. Butler, Montgomery C. Meigs, Joseph Hooker, and David D. Porter Library of Congress Cataloging in Publication Data Library of Congress. Manuscript Division. Civil War manuscripts. Includes index. Supt. of Docs, no.: LC 42:C49 1. United States—History—Civil War, 1861-1865— Manuscripts—Catalogs. 2. United States—History— Civil War, 1861-1865—Sources—Bibliography—Catalogs. 3. Library of Congress. Manuscript Division—Catalogs. I. Sellers, John R. II. Title. Z1242.L48 1986 [E468] 016.9737 81-607105 ISBN 0-8444-0381-4 The portraits in this guide were reproduced from a photograph album in the James Wadsworth family papers, Manuscript Division, Library of Congress. The album contains nearly 200 original photographs (numbered sequentially at the top), most of which were autographed by their subjects. The photo- graphs were collected by John Hay, an author and statesman who was Lin- coln's private secretary from 1860 to 1865. For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402. PREFACE To Abraham Lincoln, the Civil War was essentially a people's contest over the maintenance of a government dedi- cated to the elevation of man and the right of every citizen to an unfettered start in the race of life. President Lincoln believed that most Americans understood this, for he liked to boast that while large numbers of Army and Navy officers had resigned their commissions to take up arms against the government, not one common soldier or sailor was known to have deserted his post to fight for the Confederacy. -

What's Inside
13 VOLUME 16 PUBLISHED by J. RUSSELL JINISHIAN TH COMPLIMENTARY ANNIVERSARY ISSUE ™ An Insider’s Guide to Marine Art for Collectors and Historians What’s Inside: • Latest News from Today’s Premier Marine Artists • Latest Marine Art Sales and Prices • Insights into the Art Market at Large • Marine Art Exhibitions Across the Country • Upcoming Auctions • Book Reviews Two Distinguished Artists Paint Historic Nantucket… Being sold to benefit the Egan Maritime Institute in Nantucket, Massachusetts John Stobart (b. 1929) Nantucket Whalers, Nantucket Harbor, 1835 Oil on Canvas 12”x 20” $125,000 Roy Cross (b.1924) Old Nantucket in the early 1840’s Whaleship Alpha Oil on Canvas 24”x 36” $55,000 Information on purchasing the artwork pictured in the MARINE ART NEWS may be obtained by contacting the Publisher, J. Russell Jinishian at (203) 259-8753 or [email protected] News From the Artists s always there are a great many exhi- Coast. In 2013 John Stobart helped kick off Across the Pond the Royal Society of Marine bitions, artistic projects and marine the exhibition with a presentation on his career. Artists, founded just after the Second World goings-on across the country and 2015’s featured artist will be Washington state War, operates its own campaign to identify and Aaround the world to report—so let’s get right resident Frank Gaffney. (www.coosart.org) reward young artists in the field. Their annual to the news. Every year the “Fellows” of the Society get get together is held in conjunction each year The American Society of Marine Artists together and review portfolio submissions with their Annual Exhibition during the month Mall Galleries (ASMA), following its mission “to recognize of artists for new members in the Society.