APG III: Bibliographical Information and Synonymy of Magnoliidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bulletin / New York State Museum
Juncaceae (Rush Family) of New York State Steven E. Clemants New York Natural Heritage Program LIBRARY JUL 2 3 1990 NEW YORK BOTANICAL GARDEN Contributions to a Flora of New York State VII Richard S. Mitchell, Editor Bulletin No. 475 New York State Museum The University of the State of New York THE STATE EDUCATION DEPARTMENT Albany, New York 12230 NEW YORK THE STATE OF LEARNING Digitized by the Internet Archive in 2017 with funding from IMLS LG-70-15-0138-15 https://archive.org/details/bulletinnewyorks4751 newy Juncaceae (Rush Family) of New York State Steven E. Clemants New York Natural Heritage Program Contributions to a Flora of New York State VII Richard S. Mitchell, Editor 1990 Bulletin No. 475 New York State Museum The University of the State of New York THE STATE EDUCATION DEPARTMENT Albany, New York 12230 THE UNIVERSITY OF THE STATE OF NEW YORK Regents of The University Martin C. Barell, Chancellor, B.A., I. A., LL.B Muttontown R. Carlos Carballada, Vice Chancellor , B.S Rochester Willard A. Genrich, LL.B Buffalo Emlyn 1. Griffith, A. B., J.D Rome Jorge L. Batista, B. A., J.D Bronx Laura Bradley Chodos, B.A., M.A Vischer Ferry Louise P. Matteoni, B.A., M.A., Ph.D Bayside J. Edward Meyer, B.A., LL.B Chappaqua Floyd S. Linton, A.B., M.A., M.P.A Miller Place Mimi Levin Lieber, B.A., M.A Manhattan Shirley C. Brown, B.A., M.A., Ph.D Albany Norma Gluck, B.A., M.S.W Manhattan James W. -

Evolutionary Consequences of Dioecy in Angiosperms: the Effects of Breeding System on Speciation and Extinction Rates
EVOLUTIONARY CONSEQUENCES OF DIOECY IN ANGIOSPERMS: THE EFFECTS OF BREEDING SYSTEM ON SPECIATION AND EXTINCTION RATES by JANA C. HEILBUTH B.Sc, Simon Fraser University, 1996 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA July 2001 © Jana Heilbuth, 2001 Wednesday, April 25, 2001 UBC Special Collections - Thesis Authorisation Form Page: 1 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. The University of British Columbia Vancouver, Canada http://www.library.ubc.ca/spcoll/thesauth.html ABSTRACT Dioecy, the breeding system with male and female function on separate individuals, may affect the ability of a lineage to avoid extinction or speciate. Dioecy is a rare breeding system among the angiosperms (approximately 6% of all flowering plants) while hermaphroditism (having male and female function present within each flower) is predominant. Dioecious angiosperms may be rare because the transitions to dioecy have been recent or because dioecious angiosperms experience decreased diversification rates (speciation minus extinction) compared to plants with other breeding systems. -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -

System Garden Masterplan, Melbourne University 2018
SYstem GARDEN LANDSCAPE MASTERPLAN STAGE 4 - MASTERPLAN FINAL REPORT 8th MARCH 2018 landscape architecture and GLAS urban design CONTENTS EXECUTIVE SUMMARY 1 INTRODUCTION 3 HistorY OF THE SYstem GARDEN 4 THE GARDEN TODAY 5 KEY ISSUES FACING THE SYstem GARDEN 6 Masterplan VISION 7 K EY VALUES 8 THE SYstem GARDEN AND OC21 9 VISION: A BOTANIC GARDEN FOR THE CAMPUS 10 MASTERPLAN PRINCIPLES 11 THE SYstem GARDEN MASTERPLAN 13 strategic INITIATIVES 15 BotanicAL DivERSiTy - SuB-cLASS PLANTiNG GuiDELiNES 16 INTERPRETATION StrateGY 17 UNIVERSITY HISTORY 18 INDIGENOUS ConnecTION 19 SUSTAINABILITY 20 MATERIALS PALETTE 21 MATERiALS PALETTE - LiGHTiNG AND PoWER 22 MATERiALS PALETTE - coNSoLiDATiNG SERvicES 23 FURNITURE 24 Access 25 ART AND EVENTS IN THE GARDEN 26 Masterplan ELEMENTS 27 master PLAN ELEMENTS 28 PERIMETER PATH AND EDGE SPACES 29 SYstem GARDEN GATEs 30 ENTRy AvENuES - BiZARRE SENTRiES 31 THE FORMAL GARDEN 32 WETLAND cANAL 37 THE INFORMAL GARDEN 38 COURTYARD GARDENS 43 rainforest GARDEN 44 FERN AND LICHEN COURTYARD 45 APOTHECARY GARDEN 47 RESEARCH GARDENS 48 implementation STAGING 50 APPENDIX 1: costing 55 APPENDIX 2: CONSULTANT REPORTS 57 EXecUTIVE SUMMARY IntroDUction The System Garden is a special space. Originally laid out in 1856 by Professor Frederick McCoy and The Core values, are key to the current and future operation of the Parkville campus, they have a • indigenous connection: the System Garden provides indigenous interpretation through Edward LaTrobe Bateman, it is a botanic garden configured specifically for learning. It provides a direct link to the University’s OC21 strategy (Our Campus in the 21st Century) and will drive the the Billibellary’s walk and stop within the System Garden. -

Appendices, Glossary
APPENDIX ONE ILLUSTRATION SOURCES REF. CODE ABR Abrams, L. 1923–1960. Illustrated flora of the Pacific states. Stanford University Press, Stanford, CA. ADD Addisonia. 1916–1964. New York Botanical Garden, New York. Reprinted with permission from Addisonia, vol. 18, plate 579, Copyright © 1933, The New York Botanical Garden. ANDAnderson, E. and Woodson, R.E. 1935. The species of Tradescantia indigenous to the United States. Arnold Arboretum of Harvard University, Cambridge, MA. Reprinted with permission of the Arnold Arboretum of Harvard University. ANN Hollingworth A. 2005. Original illustrations. Published herein by the Botanical Research Institute of Texas, Fort Worth. Artist: Anne Hollingworth. ANO Anonymous. 1821. Medical botany. E. Cox and Sons, London. ARM Annual Rep. Missouri Bot. Gard. 1889–1912. Missouri Botanical Garden, St. Louis. BA1 Bailey, L.H. 1914–1917. The standard cyclopedia of horticulture. The Macmillan Company, New York. BA2 Bailey, L.H. and Bailey, E.Z. 1976. Hortus third: A concise dictionary of plants cultivated in the United States and Canada. Revised and expanded by the staff of the Liberty Hyde Bailey Hortorium. Cornell University. Macmillan Publishing Company, New York. Reprinted with permission from William Crepet and the L.H. Bailey Hortorium. Cornell University. BA3 Bailey, L.H. 1900–1902. Cyclopedia of American horticulture. Macmillan Publishing Company, New York. BB2 Britton, N.L. and Brown, A. 1913. An illustrated flora of the northern United States, Canada and the British posses- sions. Charles Scribner’s Sons, New York. BEA Beal, E.O. and Thieret, J.W. 1986. Aquatic and wetland plants of Kentucky. Kentucky Nature Preserves Commission, Frankfort. Reprinted with permission of Kentucky State Nature Preserves Commission. -

DDC) Stemming from the Adoption of the APG (Angiosperm Phylogeny Group) III Classification As the Basis for the DDC’S Treatment of Flowering Plants
This PDF documents proposed changes throughout the Dewey Decimal Classification (DDC) stemming from the adoption of the APG (Angiosperm Phylogeny Group) III classification as the basis for the DDC’s treatment of flowering plants. We request comment from any interested party, to be sent to Rebecca Green ([email protected]) by 31 January 2016. Please include “Angiosperm review comments” in your subject line. -------------------------------------------------------------- Why is the DDC adopting a new basis for classifying angiosperms (flowering plants)? During the latter half of the 20th century, biological classification turned from establishing taxa predominantly on the basis of morphological similarities to establishing taxa predominantly on the basis of shared ancestry / shared derived characters, with biological taxonomies mirroring evolutionary relationships. Phylogenetic analysis typically underlies modern evolutionary classifications, but has resulted in the development of many competing classifications. Within the domain of flowering plants, different classification systems have been favored in different countries. The Angiosperm Phylogeny Group, a global consortium of botanists, has addressed this issue by developing a “consensus” classification that is monophyletic (i.e., its taxa include all but only the descendants of a common ancestor). Now in its third version, the APG III classification is considered relatively stable and useful for both research and practice (e.g., for organizing plants in herbaria). The development for flowering plants presented here is the culmination of DDC editorial work over a span of several years. An early version revised 583–584 to make the schedule compatible with the APG III classification, while trying to minimize relocations and using see references to establish the APG III logical hierarchy. -
Comparison of Cronquist and Phylogeneticalt
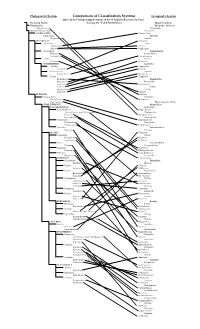
Phylogenetic System Comparison of Classification Systems Cronquist's System Lines Show Corresponding Positions of the 60 Families Reviewed in Class Flowring Plants Biology 211 - Fall 2002 (Phillips) Magnoliophyta Nymphaeales Liliopsida - Monocots Nymphaeaceae Alismatidae (unnamed major clade) Alismatales MAGNOLIIDS Alismataceae Magnoliales Arecidae Magnoliaceae Arecales MONOCOTS Arecaceae Alismatales Arales Alismataceae Araceae Araceae (incl. Lemnaceae) Lemnaceae Asparagales Commelinidae Iridaceae Commelinales Orchidaceae Commelinaceae Liliales Juncales Liliaceae Juncaceae COMMELINIDS Cyperales Arecales Cyperaceae Arecaceae Poaceae Commelinales Typhales Commelinaceae Sparganiaceae Poales Typhaceae Bromeliaceae Zingiberidae Cyperaceae Bromeliales Juncaceae Bromeliaceae Poaceae Lilidae Sparganiaceae Liliales Typhaceae Liliaceae EUDICOTS Iridaceae Ranunculales Orchidales Ranunculaceae Orchidaceae Papaveraceae Magnoliopsida - Dicots CORE EUDICOTS Magnoliidae Caryophyllid Clade Magnoliales Caryophyllales Magnoliaceae Amaranthaceae (incl. Chenopodiaceae) Nymphaeales Cactaceae Nymphaeaceae Caryophyllaceae Ranunculales Polygonales Ranunculaceae Droseraceae Papaverales Polygonaceae Papaveraceae Santalales Hamamelididae Viscaceae Urticales ROSIDS Ulmaceae Saxifragales Fagales Crassulaceae Fagaceae Saxifragaceae Betulaceae Vitales Caryophyllidae Vitaceae Caryophyllales Geraniales Cactaceae Geraniaceae Chenopodiaceae Myrtales Caryophyllaceae Onagraceae Polygonales EUROSIDS I Polygonaceae Cucurbitales Dilleniidae Cucurbitaceae Malvales Fabales Tiliaceae -

An Evolutionary Particularity Principle for Evolutionary System of Classes of Fructophyta
American Journal of Agriculture and Forestry 2019; 7(5): 191-199 http://www.sciencepublishinggroup.com/j/ajaf doi: 10.11648/j.ajaf.20190705.15 ISSN: 2330-8583 (Print); ISSN: 2330-8591 (Online) An Evolutionary Particularity Principle for Evolutionary System of Classes of Fructophyta Da-Li Fu 1, 2, * 1Non-timber Forestry Research and Development Center, Chinese Academy of Forestry, Zhengzhou, China 2Key Laboratory of Non-timber Forest Germplasm Enhancement & Utilization of National Forestry and Grassland Administration, Zhengzhou, China Email address: To cite this article: Da-Li Fu. An Evolutionary Particularity Principle for Evolutionary System of Classes of Fructophyta. American Journal of Agriculture and Forestry . Special Issue: The New Evolutionary Theory & Practice . Vol. 7, No. 5, 2019, pp. 191-199. doi: 10.11648/j.ajaf.20190705.15 Received : August 23, 2019; Accepted : August 28, 2019; Published : September 20, 2019 Abstract: Fructophyta D. L. Fu & H. Fu, a new division established in 2018, including all fruit or flowering plants, conventionally named as angiosperms, occupy the highest evolutionary phylum taxa and an important position in terrestrial ecosystems and human wellbeing, whose origin and evolution had always been thought as puzzling. To scientifically settle the puzzle and using the evolutionary continuity principle of new science of Evolutionomy, the author proposed that evolutionary taxa should have particularly evolutionary characters to be distinguished to the closer taxa, which could be called evolutionary particularity principle. Based on the principle, the evolutionary system of five classes of Fructophyta D. L. Fu & H. Fu can be affirmed, which is a system of dichotomous evolution. Two new classes, Leguminopsida D. -

Global Grass (Poaceae) Success Underpinned by Traits Facilitating Colonization, Persistence and Habitat Transformation
This is a repository copy of Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/129542/ Version: Accepted Version Article: Linder, H.P., Lehmann, C.E.R., Archibald, S. et al. (2 more authors) (2017) Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biological Reviews, 93 (2). pp. 1125-1144. ISSN 1464-7931 https://doi.org/10.1111/brv.12388 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation H. Peter Linder1,*, Caroline E.R. Lehmann2,3, Sally Archibald3, Colin P. Osborne4 and David M. Richardson5 1Department of Systematic and Evolutionary Botany, -

Flora of the Guianas Project
Leiden, October 2012 The Flora of the Guianas is a co-operative programme of: Botanischer Garten und Botanisches Museum Berlin-Dahlem, Berlin; Institut de Recherche pour le Développement, IRD, Centre de Cayenne, Cayenne; Department of Biology, University of Guyana, Georgetown; Herbarium, Royal Botanic Gardens, Kew; New York Botanical Garden, New York; Nationaal Herbarium Suriname, Paramaribo; Muséum National d’Histoire Naturelle, Paris; Nationaal Herbarium Nederland, Utrecht University branch, Utrecht, and Department of Botany, Smithsonian Institution, Washington, D.C. For further information see the website: http://www.nationaalherbarium.nl/FoGWebsite/index.html Published on June 2014 Flora of the Guianas Newsletter no 18. Nationaal Herbarium Nederland, Naturalis Biodiversity Center, Einsteiweg 2, 2333 CC, Leiden, The Netherlands 2 CONTENTS In Memorian M.F.Prévost, J. Florschutz 1. MEETING PROGRAM ...................................................................................................... 6 2. MINUTES OF THE ADVISORY BOARD MEETING .............................................................. 7 2.1. Opening and report on previous meeting in Washigton .............................................. 7 2.2. Board personnel changes ............................................................................................ 7 2.3. Memorandum of Understanding ................................................................................. 7 2.4. Report by the executive editor ................................................................................... -

Year Sem. Subject Code Title of the Paper Hours/ Week 2018 -2019 Onwards III 18MBO31C PAPER VII PLANT SYSTEMATICS, RESOURCES A
Year Sem. Subject Code Title of the paper Hours/ Week III 18MBO31C PAPER VII PLANT SYSTEMATICS, RESOURCES 7 2018 -2019 AND ETHNOBOTANY onwards Objectives: 1. To acquire the fundamental values of plant systematics 2. To know about the basic concepts and principles of plant systematics 3. To establish a suitable method for correct identification and adequate characterization of plants 4. To aware of the importance of taxonomic relationships in plant systematic studies 5. To understand the utility of different plant species 6. To have a first- hand knowledge on Economic Botany and Ethnobotany Unit – I History of classification; Systems of classification: Bentham and Hooker and Cronquist; Angiosperm Phylogeny Group 2011; International Code for Botanical Nomenclature; Typification, Valid publication, Citation, Retention choice and Rejection of names; Priority. Unit –II Plant molecular systematics; Chemotaxonomy and Numerical taxonomy; Taxonomic evidences from Morphology, Anatomy, Embryology, Palynology and Cytology; Concepts of Taxa and Taxonomic hierarchy; Construction and uses of different types of key for plant identification (indented and bracket keys); Basic concepts of Flora, Revisions, Monographs, Herbaria and Data information system; Botanical Gardens. Unit – III Comparative and detailed study of the following families: Nymphaceae, Capparidaceae Polygalaceae, Portulacaceae, Zygophyllaceae, Rhamnaceae, Sapindaceae, Combretaceae, Passiflorae, Ebenaceae, Ficodeae, Rubiaceae, Oleaceae and Boraginaceae. Unit – IV Comparative and detailed -
Morphology of Hydatellaceae, an Anomalous Aquatic Family Recently Recognized As an Early-Divergent Angiosperm Lineage1
American Journal of Botany 94(7): 1073–1092. 2007. MORPHOLOGY OF HYDATELLACEAE, AN ANOMALOUS AQUATIC FAMILY RECENTLY RECOGNIZED AS AN EARLY-DIVERGENT ANGIOSPERM LINEAGE1 PAULA J. RUDALL,2,8 DMITRY D. SOKOLOFF,3 MARGARITA V. REMIZOWA,3 JOHN G. CONRAN,4 JERROLD I. DAVIS,5 TERRY D. MACFARLANE,6 AND DENNIS W. STEVENSON7 2Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; 3Department of Higher Plants, Biological Faculty, Moscow State University, 119992, Moscow, Russia; 4CEBB, EB/EES, Benham Building, DP312, University of Adelaide, Adelaide, SA 5005, Australia; 5L. H. Bailey Hortorium and Department of Plant Biology, Cornell University, Ithaca, New York 14853 USA; 6CALM, c/o Manjimup Research Centre, Brain Street, 6258 Manjimup, WA, Australia; and 7New York Botanical Garden, Bronx, New York 10458 USA The family Hydatellaceae was recently reassigned to the early-divergent angiosperm order Nymphaeales rather than the monocot order Poales. This dramatic taxonomic adjustment allows comparison with other early-divergent angiosperms, both extant and extinct. Hydatellaceae possess some monocot-like features that could represent adaptations to an aquatic habit. Ecophysiological parallels can also be drawn from fossil taxa that are known from small achene-like diaspores, as in Hydatellaceae. Reproductive units of Hydatellaceae consist of perianthlike bracts enclosing several pistils and/or stamens. In species with bisexual reproductive units, a single unit resembles an ‘‘inside-out’’ flower, in which stamens are surrounded by carpels that are initiated centrifugally. Furthermore, involucre development in Trithuria submersa, with delayed growth of second whorl bracts, resembles similar delayed development of the second perianth whorl in Cabomba. Several hypotheses on the homologies of reproductive units in Hydatellaceae are explored.