The Kinetics of Nitrogen Oxides Formation in the Flame Gas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2013 KIVA Development
2013 DOE Merit Review 2013 KIVA Development David Carrington Los Alamos National Laboratory May 13, 2013 Project ID # ACE014 This presentation does not contain any proprietary, confidential, or otherwise restricted information LA-UR-13-21976 2013 DOE Overview Merit Review Timeline Barriers • Improve understanding of the fundamentals of • 10/01/09 fuel injection, fuel-air mixing, thermodynamic combustion losses, and in-cylinder combustion/ • 09/01/14 emission formation processes over a range of combustion temperature for regimes of interest • 65% complete by adequate capability to accurately simulate these processes • Engine efficiency improvement and engine- Budget out emissions reduction • Minimization of engine technology development • Total project funding to date: – User friendly (industry friendly) software, robust, accurate, more predictive, & quick meshing – 2000K – 640K in FY 12 Partners – Contractor (Universities) share ~40% • University of New Mexico- Dr. Juan Heinrich • University of Purdue, Calumet - Dr. Xiuling • Funding to date for FY13 - 210K Wang • Funding anticipated FY13 – 763K • University of Nevada, Las Vegas - Dr. Darrell W. Pepper 2 2013 DOE FY 09 to FY 14 KIVA-Development Merit Review Objectives • Robust, Accurate Algorithms in a Modular Object-Oriented code– • Relevance to accurately predicting engine processes to enable better understanding of, flow, thermodynamics, sprays, in easy to use software for moderate computer platforms – More accurate modeling requires new algorithms and their correct implementation. – Developing more robust and accurate algorithms • To understand better combustion processes in internal engines – Providing a better mainstay tool • improving engine efficiencies and • help in reducing undesirable combustion products. – Newer and mathematically rigorous algorithms will allow KIVA to meet the future and current needs for combustion modeling and engine design. -

Catalytically Generating and Utilizing Hydrogen to Reduce Nox Emissions in Automobile Applications
Catalytically Generating and Utilizing Hydrogen to Reduce NOx Emissions in Automobile Applications Thesis by Nawaf Mohammed Alghamdi In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia November, 2018 2 EXAMINATION COMMITTEE PAGE The thesis of Nawaf Mohammed Alghamdi is approved by the examination committee. Committee Chairperson: Professor Mani Sarathy Committee Members: Professor Jorge Gascon and Professor Aamir Farooq 3 © November, 2018 Nawaf Mohammed Alghamdi All Rights Reserved 4 ABSTRACT Catalytically Generating and Utilizing Hydrogen to Reduce NOx Emissions in Automobile Applications Nawaf Mohammed Alghamdi Heterogeneous catalysis is a powerful chemical technology because it can enhance the conversion of reactants, promote selectivity to a desired product, and lower the reaction temperature requirements. The breaking and forming of chemical bonds in heterogeneous catalysis is facilitated on a solid surface where adsorbed gas-phase species react and form products. This study is concerned with utilizing heterogeneous catalysis in the automobile industry via the generation and utilization of hydrogen to reduce NOx emissions. In spark ignition engines, the three-way-catalyst technology is ineffective at the more efficient, lean-burn conditions. In compression-ignition engines, an ammonia-based technology is implemented but has associated high cost and ammonia slip challenges. This motivates providing an alternative technology, such as hydrogen selective catalytic reduction (H2- SCR). In this study, four catalysts were investigated for the lean-burn selective catalytic reduction of NO using hydrogen. The catalysts were platinum (Pt) and palladium (Pd) noble metals supported on cerium oxide (CeO2) and magnesium oxide (MgO). -

Combustion Research Facility – Industry Interactions and Impact
Combustion Research Facility – Industry Interactions and Impact Bob Hwang Director, Transportation Energy Center Sandia National Laboratories, Livermore, CA Sandia Sites Albuquerque, New Mexico Livermore, California Kauai, Hawaii Pantex Plant, Amarillo, Texas Waste Isolation Pilot Plant, Tonopah, Carlsbad, New Mexico Nevada 2 CRF - Understanding Combustion Processes A Clearly Defined Partnership Mission from the Beginning • Born out of gasoline crises of 1970’s – created in 1980 • Built to tap into strengths of existing NNSA laboratory – Premier optical diagnostics – High Performance Computing – Flagship experimental facilities • Teaming at DOE – Office of Science unwavering “Do great science” mandate • “Basic Energy Sciences (BES) supports fundamental research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security.” – Vehicle Technologies partnership: “make an impact” • “The U.S. Department of Energy's Vehicle Technologies Office supports research, development (R&D), and deployment of efficient and sustainable highway transportation technologies to improve vehicles’ fuel economy and minimize petroleum use.” • Strong industrial and academic ties from day #1 Combustion Research Facility A DOE Collaborative Research Facility dedicated to energy science and technology for the twenty-first century • Leadership in combustion research since 1980 • 8200-m2 -
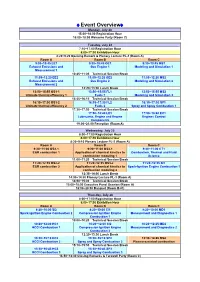
Comodia 2012
♦Event Overview♦ Monday, July 23 15:00~18:00 Registration Hour 16:00~18:00 Welcome Party (Room C) Tuesday, July 24 7:10~17:00 Registration Hour 8:00~17:30 Exhibition Hour 8:20~9:20 Opening Remark & Plenary Lecture PL-1 (Room A) Room A Room B Room C 9:30~10:45 EE1 9:30~10:45 GE1 9:30~10:45 MS1 Exhaust Emissions and Gas Engine 1 Modeling and Simulation 1 Measurement 1 10:45~11:05 Technical Session Break 11:05~12:20 EE2 11:05~12:20 GE2 11:05~12:20 MS2 Exhaust Emissions and Gas Engine 2 Modeling and Simulation 2 Measurement 2 12:20~13:50 Lunch Break 13:50~15:55 OS1-1 13:50~15:55 FL1 13:50~15:55 MS3 Ultimate thermal efficiency 1 Fuels 1 Modeling and Simulation 3 15:55~16:15 Technical Session Break 16:15~17:30 OS1-2 16:15~17:30 FL2 16:15~17:30 SP1 Ultimate thermal efficiency 2 Fuels 2 Spray and Spray Combustion 1 17:30~17:50 Technical Session Break 17:50~18:40 LE1 17:50~18:40 EC1 Lubricants, Engine and Engine Engines Control Components 19:00~21:00 Reception (Room A) Wednesday, July 25 8:00~17:00 Registration Hour 8:00~17:30 Exhibition Hour 8:20~9:10 Plenary Lecture PL-2 (Room A) Room A Room B Room C 9:20~11:00 OS2-1 9:20~11:00 OS3-1 9:20~11:00 CT1 EGR combustion 1 Application of chemical kinetics to Combustion, Thermal and Fluid combustion modeling 1 Science 11:00~11:20 Technical Session Break 11:20~12:35 OS2-2 11:20~12:35 OS3-2 11:20~12:35 SI1 EGR combustion 2 Application of chemical kinetics to Spark-Ignition Engine Combustion 1 combustion modeling 2 12:35~14:00 Lunch Break 14:00~14:50 Plenary Lecture PL-3 (Room A) 14:50~15:00 Technical Session -

VALVE CONTROLS INFINITE POSSIBILITIES ROGER STONE, CAMCON AUTOMOTIVE TECHNICAL DIRECTOR Where All the Pieces Fall Into Place
The pan-European magazine of SAE International May/June 2013 VALVE CONTROLS INFINITE POSSIBILITIES ROGER STONE, CAMCON AUTOMOTIVE TECHNICAL DIRECTOR Where All the Pieces Fall Into Place Let’s face it; driveline design can be a real brainteaser. Reduced emissions. Improved fuel economy. Enhanced vehicle performance. Getting all the pieces to fit is challenging—especially when you can’t see the whole picture. That’s why you need one more piece to solve the driveline puzzle. Introducing DrivelineNEWS.com—a single, all-encompassing website exclusively dedicated to driveline topics for both on- and off-road vehicles. Log on for the latest news, market intelligence, technical innovations and hardware animations to help you find solutions and move your business forward. It takes ingenuity and persistence to succeed in today’s driveline market. Test your skills at DrivelineNEWS.com/Puzzle. Solve the puzzle to enter to win a Kindle Fire. © 2013 The Lubrizol Corporation. All rights reserved. 121569 Contents 12 12 Cover Story 5 Comment Valve controls Imagine a valve control system that is infinitely 6 variable irrespective of engine speed and load. News Impossible? Not according to Camcon Automotive’s • Exclusive: technical director Roger Stone, as Ian Adcock Engine knock discovers predicted 16 Biomimicry • Volvo diesel breakthrough Mother Nature knows best What can the automotive industry learn from nature • Driveline software when it comes to weight saving? Ryan Borroff has 20 been finding out • Four-way cat for petrol engines 20 Fuel injection systems Building up the pressure 11 Tony Lewin reports on the growing trend towards even The Columnist higher line pressures in injection systems. -

Modeling and Simulation of a Free-Piston Engine with Electrical Generator Using HCCI Combustion
Graduate Theses, Dissertations, and Problem Reports 2016 Modeling and Simulation of a Free-Piston Engine with Electrical Generator Using HCCI Combustion Mohammad Alrbai Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Alrbai, Mohammad, "Modeling and Simulation of a Free-Piston Engine with Electrical Generator Using HCCI Combustion" (2016). Graduate Theses, Dissertations, and Problem Reports. 5086. https://researchrepository.wvu.edu/etd/5086 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Modeling and Simulation of a Free-Piston Engine with Electrical Generator Using HCCI Combustion ____________________________________________ Mohammad Alrbai Dissertation Submitted to the Benjamin M. Statler College of Engineering and Mineral Resources at West Virginia University in partial fulfillment of the requirements for the degree of Doctorate of Philosophy in Mechanical Engineering Nigel Clark, Ph.D., Chair Hailin Li, Ph.D. Cosmin Dumitrescu, Ph.D. Terence Musho, Ph.D. Fernando V. Lima, Ph.D. Department of Mechanical and Aerospace Engineering Morgantown, West Virginia 2016 Keywords: Free Piston Engine, Electrical Generator, Chemical kinetics, HCCI Combustion, Model Control, EGR, Equivalence Ratio. -

12Th Diesel Engine-Efficiency and Emissions Research (DEER
7/28/06 3 p.m. 12th Diesel Engine-Efficiency and Emissions Research Conference Detroit Marriott at the Renaissance Center August 20-24, 2006 ---------- PLENARY SESSIONS ---------- Monday, August 21, 2006 8:30 a.m. - Noon A View from the Bridge Panel Discussion Andrew Karsner, Assistant Secretary for Energy Efficiency and Renewable Energy at the U.S. Department of Energy Elizabeth Lowery, Vice-President for Environment and Energy at General Motors Gerhard Schmidt, Vice-President for Research and Advanced Engineering at Ford Motor Company Margo Ogé, Director of the Office of Transportation and Air Quality at the U.S. Environmental Protection Agency (EPA) John K. Amdall, Director of Engine Research and Development at Caterpillar Tom Cackette, Chief Deputy Executive Officer at the California Air Resources Board Michael Walsh, environmental consultant Tuesday, August 22, 2006 8:30 a.m. - Noon Accelerating Light-Duty Diesel Sales in the U.S. Market Panel Discussion Charles Freese, General Motors Simon Godwin, DaimlerChrysler Kevin McMahon, Martec Group Karl Simon, EPA Wolfgang Mattes, BMW Yasuyuki Sando, Senior Manager, Advanced Engine Research Division at Honda Klaus-Peter Schindler, Volkswagen 1 Wednesday, August 23, 2006 8:30 a.m. - Noon New Feedstocks and Replacement Fuels Panel Discussion Loren Beard, DaimlerChrysler (invited) Norman Brinkman, General Motors Nigel Clark, West Virginia University Herb Dobbs, TACOM Craig Fairbridge, National Centre for Upgrading Technology Robert McCormick, National Renewable Energy Laboratory James Simnick, BP ________ TECHNICAL SESSIONS _______ Monday, August 21, 2006 1:30 – 4:10 p.m. Technical Session 1 – Advanced Combustion Technologies, Part 1 Title Speaker Affiliation Heavy-Duty HCCI Development Kevin Duffy Caterpillar Activities at Caterpillar Low-Temperature Combustion for Dennis Assanis University of High-Efficiency, Ultra-Low Emission Michigan Engines Evaluation of High-Efficiency Clean Robert M. -

Oak Ridge National Laboratory Capabilities
FEMP Team Capabilities at ORNL in Buildings and Fleets Managed by UT-Battelle for the Department of Energy ORNL FEMP Team — Primary Work-for-Others Technical Assistance Capabilities Customers • Geothermal (ground-source) heat pumps • DOE • Combined heat and power (CHP, • US Navy distributed energy, cogeneration) • Marine Corps • Benchmarking and strategic energy • US Army management • Air Force • Industrial energy efficiency • Coast Guard • Energy assessments • Environmental Protection Agency • Alternative financing • Housing and Urban • Measurement & Development verification • Fish and Wildlife Service • Commissioning, O&M • Tennessee Valley Authority • Federal design/construction practices • State of California • General technical/design assistance • New York State ERDA – LEED, Energy Star, life-cycle cost, modeling, etc. 2 Managed by UT-Battelle for the Department of Energy 1 Geothermal Heat Pumps — Retrofits or New Construction ORNL’s independent design review and simulation modeling for residential GHP • Feasibility studies systems for Marine Corps Air Station Beaufort, South Carolina (“Fightertown”) • Life-cycle-cost studies contributed to the success of the project. • Review of technical and price proposals • Review of system design • Interpretation of thermal properties tests • Review of bore field sizing • Baseline and energy savings estimates/ calculations • Review of pricing • Development of building simulation models • Development of measurement and verification plans 3 Managed by UT-Battelle for the Department of Energy Presentation_name -

SAE 2011 World Congress & Exhibition
SAE 2011 World Congress & Exhibition Technical Session Schedule As of 04/18/2011 07:40 pm Tuesday, April 12 New Transmission Technologies That Could Change the Face of Powertrain Performance Session Code: ANN200 Room FEV Powertrain Innovation Forum Session Time: 10:00 a.m. New transmission concepts and developments are occurring in parallel with new innovations in engine technology, allowing OEMs to produce powertrain systems that meet both government regulatory mandates while still giving the consumer the drivability they have come to expect. Not only have the number of speeds increased, new types of transmissions and control strategies are coming to market. These include DCT, electrically assisted AMT, electrically assisted hybrid automatics, etc. Transmission experts on the panel will discuss new development trends, control and calibration strategies as well as provide a glimpse into the future for these new design trends. Moderators - Mircea Gradu, Director Transmission & Driveline Engrg, Chrysler Group LLC Panelists - Thomas Brown, General Mgr, Drivetrain Engrg, Aisin Tech. Center of America; Kiran Govindswamy, Chief Engineer, NVH, CAE & Driveline Systems, FEV, Inc.; Steven G. Thomas, Mgr, Transmission/Driveline, Res & Adv Engrg, Ford Motor Co.; Hamid Vahabzadeh, General Director, Global Adv Trans Engrg, GM Powertrain; Andy Yu, Vice President Engineering, BorgWarner Inc.; Tuesday, April 12 Charging Forward Toward 2017: The Powertrains That Will Get Us There Session Code: ANN201 Room FEV Powertrain Innovation Forum Session Time: 1:00 p.m. While much of the regulatory attention may be shifting to CAFE, ozone and CO2 limits proposed by both CARB and EPA for 2017 to 2025, the 2016 regulations still must be met. -

Alternative Ad.Qxd
P001_ADEU_MAY11.qxp:Layout 1 23/5/11 18:28 Page 1 The pan-European magazine of SAE International May/June 2011 Variable compression ratio reborn CFD combustion analysis Lighting the way Exhaust materials SAE Review P002_ADEU_MAY11 19/5/11 10:38 Page 1 WITHOUT DUAL CLUTCH TRANSMISSION TECHNOLOGY, YOU’RE JUST GETTING LEFT BEHIND. Maybe it’s the fact that in the time you read this, a Dual Clutch Transmission could switch gears 40,000 times. Perhaps it’s the fact that DCTs appeal to more buyers by combining impressive fuel-economy, the smooth ride of an automatic and the speed of a manual. It could be the fact that leading clutch suppliers estimate they’ll quadruple DCT sales by 2014. Or maybe it’s the fact that by 2015, 10% of all passenger cars will have them. DCTFACTS.COM gives you endless reasons to believe that DCTs are the future generation DCTFACTS.COM of transmissions. And the more you know about them, the further ahead you’ll get. P003_ADEU_MAY11.qxp:Layout 1 23/5/11 18:18 Page 3 Contents 16 16 Spotlight on Bernie Rosenthal 5 Comment Ian Adcock talks with the Reaction Design CEO After the bad.... about the company’s ground-breaking computional comes the good fluid dynamics package for combustion analysis. 6 News 22 Cover feature Laser spark sees the Consumption: The Mother of Invention light • Two eyes are It’s a challenge that has engaged the minds of many better than one • down the years: changing the compression ratio in an Avago launches first engine on the fly. -
ASME® 2018 ICEF Internal Combustion Engine Fall Technical Conference
ASME® 2018 ICEF Internal Combustion Engine Fall Technical Conference CONFERENCE November 4 – 7, 2018 EXHIBITION November 5 – 6, 2018 Hilton San Diego Resort & Spa Program San Diego, CA e American Society of Mechanical Engineers® ASME® Technical Sessions MONDAY, NOVEMBER 5 TRACK 3 ADVANCED COMBUSTION TRACK 2 FUELS Track Chair: Sundar Rajan Krishnan, University of Alabama, Tuscaloosa, AL, United States Track Chair: Gregory Bogin, Colorado School of Mines, Golden, CO, United States Track Co-Chair: Cosmin Dumitrescu, West Virginia University, Morgantown, WV, United States Track Co-Chair: Hailin Li, West Virginia University, Morgantown, WV, United States 3-1 FUNDAMENTALS/SI/THERMODYNAMICS SAN DIEGO, CA, HILTON RESORT & SPA, SORRENTO 2-1 SPARK IGNITION, FUEL SPRAYS AND IGNITION KINETICS 10:00AM - 12:00PM SAN DIEGO, CA, HILTON RESORT & SPA, LAS PALMAS 10:00AM - 12:00PM Session Chair: Hui Xu, Cummins Inc., Columbus, IN, United States Session Chair: Douglas Longman, Argonne Natl Lab., Argonne, IL, United Session Co-Chair: Joshua Bittle, University of Alabama, Tuscaloosa, AL, States United States, Tiegang Fang, NC State University, Raleigh, NC, United States, Isaac Ekoto, 7011 East avenue, Livermore, CA, United States Session Co-Chair: Scott Wayne, West Virginia University, Morgantown, WV, United States EFFECT OF DIRECT FUEL INJECTION STRATEGY ON COMBUSTION, PERFORMANCE AND EMISSIONS OF A PORT FUEL INJECTED EXPERIMENTAL INVESTIGATIONS OF HYDROGEN-NATURAL GAS COMPRESSED NATURAL GAS ENGINE USING SIMULATION TOOLS ENGINES FOR MARITIME APPLICATIONS -

Diesel Engines
p r o g r a m m E September 14th˜17th, 2010 ValencIa (SpaIn) THIESEL · 2010 conference on: Thermo-and fluid dynamic processes in Diesel Engines ORGANISED BY: CMT – MoTores TérMiCos. Universidad Politécnica de valencia, Spain Bienvenida Welcome Una vez más, un año más, La Universidad Politécnica Once more, this year again, the Universidad Politécnica de de Valencia acoge la conferencia THIESEL sobre Procesos Valencia is hosting the THIESEL Conference on Thermo-and Termofluidodinámicos en motores Diesel. Esta es la 6ª edición, Fluid Dynamic Processes in Diesel Engines. This is its 6th con lo que se puede asegurar que el proyecto está consolidado edition, which confirms that it is by now a well consolidated y aparece como cita obligada en el calendario internacional project. It has also become a must in the international de encuentros científicos sobre motores Diesel. calendar of scientific meetings about Diesel engines. Cada edición ha mostrado una particularidad, y en este caso, Each of the editions has had its own particularity. This one will be ha sido en primer lugar los acontecimientos económicos y marked on the one hand by the economical and financial events financieros que han afectado de forma especial al sector de that have especially affected the automotive sector, and on the automoción. Y por otra parte la aparición de un contexto que parece other hand, by the new trend that favours the implementation favorecer la implantación de la tracción eléctrica en los turismos. of the electrical powertrain in light-duty vehicles. Con todo ello, los que estamos en este sector, seguimos All things considered, we who work in this sector, still pensando que los motores térmicos, y en particular el motor believe that thermal engines, and in particular Diesel engines, Diesel aún tienen mucho camino por recorrer, y buena prueba have yet plenty of kilometres to travel.