Clinical Trial Details (PDF Generation Date :- Fri, 03 Sep 2021 20:08:19 GMT)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PROFILE of ANANTAPUR DISTRICT the Effective Functioning of Any Institution Largely Depends on The
PROFILE OF ANANTAPUR DISTRICT The effective functioning of any institution largely depends on the socio-economic environment in which it is functioning. It is especially true in case of institutions which are functioning for the development of rural areas. Hence, an attempt is made here to present a socio economic profile of Anantapur district, which happens to be one of the areas of operation of DRDA under study. Profile of Anantapur District Anantapur offers some vivid glimpses of the pre-historic past. It is generally held that the place got its name from 'Anantasagaram', a big tank, which means ‘Endless Ocean’. The villages of Anantasagaram and Bukkarayasamudram were constructed by Chilkkavodeya, the Minister of Bukka-I, a Vijayanagar ruler. Some authorities assert that Anantasagaram was named after Bukka's queen, while some contend that it must have been known after Anantarasa Chikkavodeya himself, as Bukka had no queen by that name. Anantapur is familiarly known as ‘Hande Anantapuram’. 'Hande' means chief of the Vijayanagar period. Anantapur and a few other places were gifted by the Vijayanagar rulers to Hanumappa Naidu of the Hande family. The place subsequently came under the Qutub Shahis, Mughals, and the Nawabs of Kadapa, although the Hande chiefs continued to rule as their subordinates. It was occupied by the Palegar of Bellary during the time of Ramappa but was eventually won back by 136 his son, Siddappa. Morari Rao Ghorpade attacked Anantapur in 1757. Though the army resisted for some time, Siddappa ultimately bought off the enemy for Rs.50, 000. Anantapur then came into the possession of Hyder Ali and Tipu Sultan. -

State: KARNATAKA Agriculture Contingency Plan for District: BIJAPUR
State: KARNATAKA Agriculture Contingency Plan for District: BIJAPUR 1.0 District Agriculture profile 1.1 Agro -Climatic/Ecological Zone Agro Ecological Sub Region Deccan Plateau, hot semi arid ecosub region ( 6.1 ) (ICAR) Agro-Climatic Region (Planning Southern Plateau and Hill Region (X) Commission) Agro Climatic Zone (NARP) Northern Dry Zone (KA-3) List all the districts or part thereof Entire District: Bijapur, Bagalkot, Gadag, Bellary, Koppal falling under the NARP Zone Part of District: Belgaum, Dharwad, Raichur, Davanagere Geographic coordinates of district Latitude Longitude Altitude 16º 49'N 75º 43'E 593 .0 m Name and address of the Regional Agricultural R esearch Station, P. B.No. 18 concerned ZRS/ ZARS/ RARS/ BIJAPUR - 586 101 RRS/ RRTTS Mention the KVK located in the district Krishi Vigyan Kendra, Bijapur 1.2 Rainfall Average (mm) Normal Onset Normal Cessation SW monsoon (June-Sep): 387.5 2 nd week of June NE Monsoon (Oct -Dec): 130 .0 4 th week of October to 4 th week of November Winter (Jan- Feb) 6.8 - - Summer (Mar-May) 56.1 - - Annual 594.4 - - 1.3 Land use Geographical Forest Land under Permanent Cultivable Land Barren and Current Other pattern of area area non- pastures wasteland under uncultivable fallows fallows the agricultural Misc. tree Land district use crops and groves Area 1053.5 2.0 35.8 9.6 5.5 1.3 29.1 85.3 5.7 (‘000 ha) 1. 4 Major Soils Area (‘000 ha) Percent (%) of total Medium black soils 401.3 40 Shallow black soils 262.5 26 Deep black soils 234.2 23 Red loamy soils 48.1 5 Red sandy soils 20.2 2 Red and -

Address of Canara Bank Wings Identified for Concurrent/ Continuous Audit by External Chartered Accountants for the Period 01St July 2021 to 30Th June 2022
ADDRESS OF CANARA BANK WINGS IDENTIFIED FOR CONCURRENT/ CONTINUOUS AUDIT BY EXTERNAL CHARTERED ACCOUNTANTS FOR THE PERIOD 01ST JULY 2021 TO 30TH JUNE 2022 S no DP Branch Name Circle Address City Pin code State Dist. 1. 6987 CPC - FT Manipal Integrated Treasury Wing, Manipal 576104 Karnataka Udupi HO Annex, II Floor, 2. 6805 CPC –FT Mumbai Canara Bank Bldgs, 8th Mumbai 400051 Maharashtra Mumbai Suburban Floor, C-14, G-Block, MCA Club, Bandra-Kurla Complex, ADDRESS OF CANARA BANK LARGE CORPORATE BRANCHES IDENTIFIED FOR CONCURRENT/ CONTINUOUS AUDIT BY EXTERNAL CHARTERED ACCOUNTANTS FOR THE PERIOD 01ST JULY 2021 TO 30TH JUNE 2022 S no DP Branch Name Circle Address City Pin code State Dist. 3. 4891 CHANDIGARH LARGE Chandigarh SCO 117-119, Sector-17C, Chandigarh 160017 Chandigarh-UT Chandigarh CORPORATE BRANCH Chandigarh 4. 2636 BENGALURU LARGE Bengaluru #18, Ramanashree Arcade, Bengaluru 560001 Karnataka Bengaluru Urban CORPORATE BRANCH III Floor, M G Road, 5. 1942 NEW DELHI Delhi II Floor, 9, World Trade Delhi 110002 NCT of Delhi-UT New Delhi CONNAUGHT PLACE Tower, Barakambha Lane, LARGE CORPORATE BRANCH 6. 19531 LARGE CORPORATE Kolkata ILLACO HOUSE, 1, Kolkata 700001 West Bengal Kolkata BRANCH KOLKATA BRABOURNE ROAD, BRABOURNE ROAD LARGE CORPORATE BRANCH, Page 1 of 45 ADDRESS OF CANARA BANK RETAIL ASSET HUBs IDENTIFIED FOR CONCURRENT/ CONTINUOUS AUDIT BY EXTERNAL CHARTERED ACCOUNTANTS FOR THE PERIOD 01ST JULY 2021 TO 30TH JUNE 2022 S no DP Branch Name Circle Address City Pin code State Dist. 7. 2284 RAH ANANTHAPUR Vijayawada Near Clock Tower, I Floor, Anantapur 515001 Andhra Pradesh Anantapur Canara Bank Branch Main-II, 8. -

The Bellary District Archaeological Project
Exploring Neolithic and Megalithic south India: the Bellary District archaeological project NICOLEBOIVIN, RAVI KORISETTAR, P.C. VENKATASUBBAIAH,HELEN LEWIS, DEEPAK HAVANUR, KALYANMALAGYANNAVAR & SUBHAS CHINCHOLI~ The southern part of the Indian peninsula is an area of the project made use of theoretical concepts of outstanding archaeological interest. While its and methodological approaches that have not historic cities and temples have long attracted the previously been applied in studies of south In- interest of both scholars and tourists, however, south dian prehistory, including symbolic and pheno- India’s equally remarkable prehistoric period remains menological approaches to understanding the have only rarely received the attention they deserve. perception and use of landscapes in the past. This A new joint Cambridge-Karnatak University re- research demonstrated that the location of sites, search project was thus initiated in 2002 to study and particularly ashmound sites, was influenced the unique Neolithic and Iron Age remains of the by patterns of visibility and movement, the pres- southern Deccan. This 2-month pilot project fo- ence of visually dramatic landscape features and cused its efforts on the Bellary District of Karnataka, the east-west movement of the sun across the sky. where prehistoric megaliths and ‘ashmounds’ It suggests that the evocative landscape of the south- (large mounds of burnt cattle dung) occupy a stun- ern Deccan was not just a backdrop for Neolithic ning landscape of naturally sculpted granitic rock activities,but rather a mythical and possibly sacred formations (FIGURE1). The aim of the project was ‘force’ that permeated many aspects of Neolithic to explore, survey and record visible archaeologi- (and subsequent Megalithicllron Age) life. -

Heritage of Mysore Division
HERITAGE OF MYSORE DIVISION - Mysore, Mandya, Hassan, Chickmagalur, Kodagu, Dakshina Kannada, Udupi and Chamarajanagar Districts. Prepared by: Dr. J.V.Gayathri, Deputy Director, Arcaheology, Museums and Heritage Department, Palace Complex, Mysore 570 001. Phone:0821-2424671. The rule of Kadambas, the Chalukyas, Gangas, Rashtrakutas, Hoysalas, Vijayanagar rulers, the Bahamanis of Gulbarga and Bidar, Adilshahis of Bijapur, Mysore Wodeyars, the Keladi rulers, Haider Ali and Tipu Sultan and the rule of British Commissioners have left behind Forts, Magnificient Palaces, Temples, Mosques, Churches and beautiful works of art and architecture in Karnataka. The fauna and flora, the National parks, the animal and bird sanctuaries provide a sight of wild animals like elephants, tigers, bisons, deers, black bucks, peacocks and many species in their natural habitat. A rich variety of flora like: aromatic sandalwood, pipal and banyan trees are abundantly available in the State. The river Cauvery, Tunga, Krishna, Kapila – enrich the soil of the land and contribute to the State’s agricultural prosperity. The water falls created by the rivers are a feast to the eyes of the outlookers. Historical bakground: Karnataka is a land with rich historical past. It has many pre-historic sites and most of them are in the river valleys. The pre-historic culture of Karnataka is quite distinct from the pre- historic culture of North India, which may be compared with that existed in Africa. 1 Parts of Karnataka were subject to the rule of the Nandas, Mauryas and the Shatavahanas; Chandragupta Maurya (either Chandragupta I or Sannati Chandragupta Asoka’s grandson) is believed to have visited Sravanabelagola and spent his last years in this place. -

Issn: 2278-6236 Multi Dimension Strategy for the Development of the Hyderabad Karnataka Region Introduction
` International Journal of Advanced Research in ISSN: 2278-6236 Management and Social Sciences Impact Factor: 6.284 MULTI DIMENSION STRATEGY FOR THE DEVELOPMENT OF THE HYDERABAD KARNATAKA REGION Dr. Ravindranath N. Kadam, Associate Professor, Dept. of Studies & Research in Economics, Kuvempu University, Shankaraghatta, Shivamogga Dist, Karnataka State, India Abstract: The sub continent, India has diversity in many respects. No doubt all the states in the country are not even in all respects. No all regions are similar and equally developed. The different Finance Commissions and the Planning commission laid stress on the objective of achieving evenhanded regional development. In Karnataka state also all the regions are not equally treated and developed. It is quite essential to maintain the equality in most of the aspects. Recently the government of Karnataka has opened eyes and showing interest in the development of backward regions of Karnataka. The development of Hyderabad Karnataka (Gulbarga Division) is to be achieved on priority base as it has been neglected by the earlier rulers (Hyderabad Nizam). The present Hyderabad Karnataka region includes Bellary, Bidar, Gulbarga, Koppal and Raichur districts. The Hyderabad Karnataka region is situated in the North-Eastern part of Karnataka and bonded with Maharashtra on the North and Andhra Pradesh on the East and South. The climate of the Hyderabad Karnataka region comprised with dryness for the major part of the year and has very hot summer and scanty rain fall. In this paper attempt is made to highlight the resources available in the Hyderabad Karnataka region and the new avenues possible for the development of the region. -

Mekala Benchi of Aspari (Kurnool District): a Unique Neolithic Site in Andhra Pradesh: a Study
Mekala Benchi of Aspari (Kurnool District): A Unique Neolithic Site in Andhra Pradesh: A Study Yadava Raghu1 1. Department of History and Archaeology, Yogi Vemana University, Kadapa – 516 005, Andhra Pradesh, India (Email: [email protected]) Received: 16 July 2019; Revised: 18 September 2019; Accepted: 24 October 2019 Heritage: Journal of Multidisciplinary Studies in Archaeology 7 (2019): 804-813 Abstract: Kurnool District in Rayalaseema region of Andhra Pradesh state is one of the richest zones of the prehistoric centers in the world and so it gives valuable information about its ancient culture. Archaeological evidences here cover a range of time periods from the present day and potentially extending back into the Pleistocene. From Captain Newbold’s time (1844) to very recent (2018) several archaeologists conducted investigations and some of them have conducted excavations in the study area. It is known on the basis of these explorations and excavations that evidence of the early man activities here are copious. In connection with the archaeological explorations I visited Mekala Benchi of Aspari, Kurnool district. In this backdrop, an attempt is made in this paper to portray the Neolithic Culture along with the rock-art depicted on the rock boulders of a hillock called boodida konda/boodidoni konda. The petro-glyphs depicted here include human figures; bulls; bulls associated with humans; elephant; horse riding; cupules and nandipadas etc. Stone tools and potsherds are also collected from the same site. Keywords: Mekala Benchi, Neolithic, Megalithic, Petroglyphs, Ethnoarchaeology, Conservation, Ceramics Introduction The food-procuring cultures were succeeded by food-producing cultures in different parts of the world. -

Status of Human Development in Hyderabad-Karnataka (HK) Region
Shivkumar, IJSRR 2019, 8(2), 4284-4293 Research article Available online www.ijsrr.org ISSN: 2279–0543 International Journal of Scientific Research and Reviews Status of Human Development in Hyderabad-Karnataka (HK) Region Shivakumar Guest Faculty, Department of Economics, Vijayanagara Sri Krishnadevaraya University, Ballari- 583105, Karnataka. Email:[email protected], Cell.9036789235 ABSTRACT: The present study analyse taluks wise Human Development and compares three dimensions of human development in inter-taluks of Hyderabad-Karnataka (HK) Region. The HK region is the one of the backward region in the state and consists of six most backward districts i.e., Bidar, Kalaburagi, Raichur, Yadgir, Ballari and Koppal that are below the state and national average in majority of socio economic indicators. The study revealedthat Bidar district with HDI value is (0.430) and Kalaburagi (0.407) districts are perform better human development in the HK region and lower the significantof HDI play in Raichur (0.196) and Yadgiri (0.165) districts in the state. Still there is insignificant variance of HDI value in entire taluks of Hyderabad-Karnataka (HK) Region KEY WORDS: HDI, Health, Education & Standard of Living Index *Corresponding author: Dr. Shivakumar Guest Faculty, Department of Economics, Vijayanagara Sri Krishnadevaraya University, Ballari-583105, Karnataka. Email:[email protected], Cell.9036789235 IJSRR, 8(2) April. – June., 2019 Page 4284 Shivkumar, IJSRR 2019, 8(2), 4284-4293 INTRODUCTION: The first Human Development Index (HDI) came in the first Human Development Report brought by United Nations Development Programme (UNDP). The HDI is designed by economist Mahbub ul Haq with the help of the conceptual framework of the capabilities approach of Amartya Sen (Sen, 1984). -

Labels Members
BDR-0001 BDR-0006 Kalyana Pattina Souharda Sahakari Ni., Gramina Mahila Multipurpose Souharda Cooperative No.9-1-65, Mohana Market Complex, 1st Floor, K.P.T.C.L Ltd. Road, Bidar - 585401 Prawarda Campus, Near Sri Beerlingeshwara Temple, Dist: Bidar Sastapura,, Basavakalyana - 585327 Ph: 08482-220559, 9901666780, 9448270104 Dist: Bidar Ph: 08481 256955, 9902410464, BDR-0008 BDR-0009 Pragathi Pattina Souharda Sahakari Ni., Kailasanatha Pattina Souharda Sahakari Ni., Mahalaxmi Plaza Complex, Gunj Bhalki, Bhalki - 585328 1st Cross, kailash Layout, Behind kailash Motors Show Dist: Bidar Room, Mannahalli Road, Bidar - 585403 Ph: 99452 76068, 99452 76068, 9449835880 Dist: Bidar Ph: 08482-234855, 8197395565, 9448119252 BDR-0010 BDR-0011 Chitaguppa Pattina Souharda Sahakari Ni., Sri Basaveshwara Souharda Pattina Sahakari Ni., Basavaraja Chouka, Main Road, Chitaguppa, Chitaguppa - Chitaguppa, Near Neharu Chouk, Main Road, Chitaguppa - 585412 585412 Dist: Bidar Dist: Bidar Ph: 08483 277407, 9611219850, 9448011382 Ph: 93432 05421, 9449455545, BDR-0014 BDR-0021 Pandith Deena Dayal Upadhyaya Pattin Souharda Srinidhi Pattina Souharda Sahakari Ni., Sahakari Ni., Amara Gangu Complex, 1st Floor, Opp. Siddarooda Mata, Plot No.50, APMC Yard, Humnabad - 585330 Mannalli Road, Bidar - 585403 Dist: Bidar Dist: Bidar Ph: 08483-270300, 9591566876, 9448018041 Ph: 08482-644555/ 8618161636/ 8867315001, 9739422310, 9844795517 BDR-0023 BDR-0025 Srinidhi Pattina Souharda Sahakari Ni. Adarsha Souharda Pattina Sahakari Ni. Near Basaveshwar Hospital, Main Road,, Basavakalyana -

Slum Free City Planning Bellary
State Urban Development Authority Government of Karnataka RAJIV AWAS YOGANA SLUM FREE CITY PLAN PLAN OF ACTION BELLARY RAJIV AWAS YOJANA SLUM FREE CITY PLAN OF ACTION BELLARY DRAFT Regional Centre for Urban and Environmental Studies Osmania University, Hyderabad Sponsored by Ministry of Urban Development, Govt. of India RAY: SLUM FREE CITY PLANNING BELLARY SLUM FREE CITY PLANNING: BELLARY Regional Centre for Urban and Environmental studies 2 RAY: SLUM FREE CITY PLANNING BELLARY CONTENTS LIST OF TABLES.......................................................................................................................... iv LIST OF CHARTS ......................................................................................................................... iv LIST OF MAPS ............................................................................................................................... v LIST OF PICTURES ..................................................................................................................... vi ACRONYMS ................................................................................................................................ viii EXECUTIVE SUMMARY............................................................................................................. xi PREAMBLE .................................................................................................................................. xii CHAPTER 1 - OVERVIEW .......................................................................................................... -
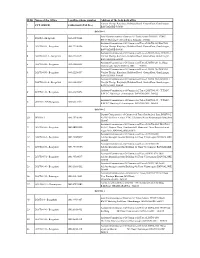
Sl.No Name of the Office Landline Phone Number Address of the Help
Sl.No Name of the Office Landline phone number Address of the help desk office Vanijya Therige Karyalaya, Kalidasa Road, Groundfloor, Gandhinagar, 1 CCT OFFICE 18004256300 (Toll Free) BANGALORE-560009 DGSTO-1 Joint Commissioner of Commercial Taxes(Admn) DGSTO1 TTMC 2 DGSTO-1 Helpdesk 080-23570264 BMTC Building Yeshwanthpura Bangalore 560022 Assistant Commissioner Of Commercial Taxes(LGSTO-10) DGSTO-1 3 LGSTO 010 - Bengaluru 080-22208556 Vanijya Therige Karyalaya, Kalidasa Road, Groundfloor, Gandhinagar, BANGALORE-560009 Assistant Commissioner Of Commercial Taxes(LGSTO-10-A) DGSTO-1 4 LGSTO 010 A - Bengaluru 080-22342691 Vanijya Therige Karyalaya, Kalidasa Road, Groundfloor, Gandhinagar, BANGALORE-560009 Assistant Commissioner Of Commercial Taxes(LGSTO-20) 3rd floor 5 LGSTO 020 - Bengaluru 080-22860622 Vishwaraiah Tower BANGALORE. 560001 Assistant Commissioner Of Commercial Taxes(LGSTO-30) DGSTO-1 6 LGSTO 030 - Bengaluru 080-22208557 Vanijya Therige Karyalaya, Kalidasa Road, Groundfloor, Gandhinagar, BANGALORE-560009 Assistant Commissioner Of Commercial Taxes(LGSTO-30A) DGSTO-1 7 LGSTO 030 A - Bengaluru 080-22342687 Vanijya Therige Karyalaya, Kalidasa Road, Groundfloor, Gandhinagar, BANGALORE-560009 Assistant Commissioner of Commercial Taxes(LGSTO-130 ) T.T.M.C. 8 LGSTO 130 - Bengaluru 080-23470478 B.M.T.C. BuildingYeshwathapura BANGALORE 560022 Assistant Commissioner of Commercial Taxes(LGSTO-130 ) T.T.M.C. 9 LGSTO 130A-Bengaluru 080-23378647 B.M.T.C. BuildingYeshwathapura BANGALORE 560022 DGSTO-2 Deputy Commissioner of Commercial Taxes(Int.Audit -

Committee on Petitions 1955-56
o. B. (I) No. 8G COMMITTEE ON PETITIONS 1955-56 SEVENTH REPORT (Presented on the 22nd December, 1955) LOK SABHA SECRETARIAT NEW DELHI January, 1956 CONTENTS PAGES 1. Members of the Committee on Petitions (i) .2. Report 1-3 3. Appendices Appendix I 7-8 Appendix II 9-10 MEMBERS OF THE COMMITTEE ON PETITIONS 1. Shri Kotha Raghuramaiah-Chairman. 2. Shri Shiva Datt Upadhyaya 3. Shri K. T. Achuthan 4. Shri Sohan Lal Dhusiya 5. Shri S. C. Deb 6. Shri Liladhar Joshi 7. Shri U. R. Bogawat 8. Shri J ethalal Harikrishna Joshi 9. Shri Ramraj J ajware 10. Shri Resham Lal Jangde 11. Shri P. N. Rajabhoj 12. Shri P. Subba Rao 13. Shri Anandchand 14. Dr. Ch. V. Rama Rao 15. Shri Ramji Verma SECRETARIAT Shri S. L. Shakdher-Joint Secretary. Shri A. L. Rai-Under SecTetClTY· :( i)' REPORT I, the Chairman of the Committee on Petitions, having been: authorised by the Committee to present the Report on their be half, present this their Seventh Report. 2. The Committee at their sitting held on the 20th December 1955 considered the following two petitions:- ' (i) Petition from the Merchants Association, Mancherial· Residents of Taluqa Asifabad, Distt. Adilabad; Villag~ Panchayat Committee, Bheemaram; Members of the· Bar, Secunderabad; Residents of Adilabad; and certain Mazdoor Unions of Warangal, relating to the report of the States Reorganisation Commission. (Petition No. 48-Appendix I). (ii) Petition from the inhabitants of Panchayat Villages in Taluk of Bellary District, My~ore, relating to the Report of the States -Reorganisation Commission. (Petition No. 49-Appendix II).