Lab Exercise: Light Absorption, Spectrophotometer, and the Pulse Oximeter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
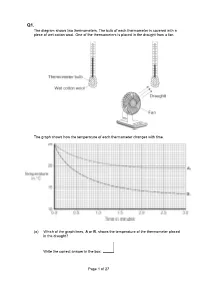
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

ADVANCED SPECTROMETER Introduction
ADVANCED SPECTROMETER Introduction In principle, a spectrometer is the simplest of scientific very sensitive detection and precise measurement, a real instruments. Bend a beam of light with a prism or diffraction spectrometer is a bit more complicated. As shown in Figure grating. If the beam is composed of more than one color of 1, a spectrometer consists of three basic components; a light, a spectrum is formed, since the various colors are collimator, a diffracting element, and a telescope. refracted or diffracted to different angles. Carefully measure The light to be analyzed enters the collimator through a the angle to which each color of light is bent. The result is a narrow slit positioned at the focal point of the collimator spectral "fingerprint," which carries a wealth of information lens. The light leaving the collimator is therefore a thin, about the substance from which the light emanates. parallel beam, which ensures that all the light from the slit In most cases, substances must be hot if they are to emit strikes the diffracting element at the same angle of inci- light. But a spectrometer can also be used to investigate cold dence. This is necessary if a sharp image is to be formed. substances. Pass white light, which contains all the colors of The diffracting element bends the beam of light. If the beam the visible spectrum, through a cool gas. The result is an is composed of many different colors, each color is dif- absorption spectrum. All the colors of the visible spectrum fracted to a different angle. are seen, except for certain colors that are absorbed by the gas. -

Funnels and Filters
51 FUNNELS AND FILTERS Buchner Funnels 260MM (10.25") INSIDE DIAMETER, 203MM (8") OVERALL HEIGHT, 127MM (5") RIM TO PLATE CATALOG NO. DESCRIPTION PRICE/EACH H14620-0000 Funnel with Coarse Porosity Fixed Plate $216.00 H14620-1260 Funnel with Coarse Porosity Removable Plate 216.00 H14625-3510 Funnel with Medium Porosity Fixed Plate 216.00 H14626-3510 Funnel with Medium Porosity Removable Plate 216.00 H14627-0000 Funnel with Perforated Fixed Plate 216.00 Table-Top H14627-1260 Funnel with Perforated Removable Plate 216.00 Buchner 457MM (18") INSIDE DIAMETER, 292MM (11.5") OVERALL HEIGHT, 203MM (8") RIM TO PLATE Funnels CATALOG NO. DESCRIPTION PRICE/EACH With Fritware® Porous H14621-0000 Funnel with Coarse Porosity Fixed Plate $540.00 Filter or HDPE H14621-1457 Funnel with Coarse Porosity Removable Plate 540.00 H14625-3518 Funnel with Medium Porosity Fixed Plate 540.00 Perforated Plate H14626-3518 Funnel with Medium Porosity Removable Plate 540.00 Polyethylene funnels are H14628-0000 Funnel with Perforated Fixed Plate 465.00 one piece design with a H14627-1457 Funnel with Perforated Removable Plate 465.00 FIXED or REMOVABLE Custom sizes available upon request. 610MM (24") INSIDE DIAMETER, 330MM (13") OVERALL HEIGHT, 267MM (10.25") RIM TO PLATE porous filter plate CATALOG NO. DESCRIPTION PRICE/EACH supported by a multiple ring grid. Filter plates are made of high density H14622-0000 Funnel with Coarse Porosity Fixed Plate $1125.00 1 polyethylene 6.4mm ( ⁄4") thick with a non-porous ring at the periphery of the plate H14622-1610 Funnel with Coarse Porosity Removable Plate 1125.00 1 H14625-3524 Funnel with Medium Porosity Fixed Plate 1125.00 which seals filter paper when used. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

Spectrum 100 Series User's Guide
MOLECULAR SPECTROSCOPY SPECTRUM 100 SERIES User’s Guide 2 . Spectrum 100 Series User’s Guide Release History Part Number Release Publication Date L1050021 A October 2005 Any comments about the documentation for this product should be addressed to: User Assistance PerkinElmer Ltd Chalfont Road Seer Green Beaconsfield Bucks HP9 2FX United Kingdom Or emailed to: [email protected] Notices The information contained in this document is subject to change without notice. Except as specifically set forth in its terms and conditions of sale, PerkinElmer makes no warranty of any kind with regard to this document, including, but not limited to, the implied warranties of merchantability and fitness for a particular purpose. PerkinElmer shall not be liable for errors contained herein for incidental consequential damages in connection with furnishing, performance or use of this material. Copyright Information This document contains proprietary information that is protected by copyright. All rights are reserved. No part of this publication may be reproduced in any form whatsoever or translated into any language without the prior, written permission of PerkinElmer, Inc. Copyright © 2005 PerkinElmer, Inc. Trademarks Registered names, trademarks, etc. used in this document, even when not specifically marked as such, are protected by law. PerkinElmer is a registered trademark of PerkinElmer, Inc. Spectrum, Spectrum 100, and Spectrum 100N are trademarks of PerkinElmer, Inc. Spectrum 100 Series User’s Guide . 3 Contents Contents............................................................................................... -

Microlab® STAR™
Microlab ® STAR™ Microlab ® STAR ™ AUTOMATED WORKFLOW SOLUTIONS CENTERED AROUND YOUR ASSAY The STAR combines Hamilton's patented pipetting technology including precise lock-and-key tip attachment, unrivaled liquid level detection, and comprehensive volume ranges to create flexible liquid handling workstations. Available in three base platform sizes, the STAR portfolio incorporates countless options to automate your workflows. Hamilton Robotics has also partnered with top leaders in the biotechnology industry to provide Standard Solutions based on commonly automated applications. Offering ready-to-start protocols for a variety of applications such as NGS, ELISA, and forensic assays, our Standard Solutions provide a faster way to automate your processes. 2 1 PATENTED TECHNOLOGY The STAR utilizes Hamilton’s proprietary Compressed O-Ring Expansion (CO-RE®) technology. CO-RE minimizes the production of aerosols and allows disposable tips or washable, steel needles to be used on channels in the same run. 2 MULTI-FUNCTIONAL ARM Our technology offers high pipetting accuracy and precision, from sub-microliter to large volumes, using Independent Channels and/or the Multi-Probe Head (MPH). Labware transportation is possible with the iSWAP® or CO-RE Grippers. The STAR can incorporate a camera, tube transportation, and other channel tools on a single arm. Comprehensive pipetting range: ■■■0.5 μL to 1 mL using the 1 mL Independent Channel ■■■50 μL to 5 mL using the 5 mL Independent Channel ■■■1 μL to 1 mL using the CO-RE 96 MPH ■■■0.1 μL to 50 μL using the CO-RE 384 MPH 3 FLEXIBLE SETUP The high-capacity deck is customized specific to your workflow, accommodating a wide range of labware and automated devices that can easily be exchanged to support multiple assays on one platform. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

Experiment 2 Radiation in the Visible Spectrum Emission Spectra Can Be
Experiment 2 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy states of the system. In the following experiments we will examine the radiation given off by sources radiating in the visible region. We will be using a spectrometer produced by Ocean Optics. Light enters the spectrometer via a fiber optic cable. Inside the spectrometer a diffraction grating diffracts the different frequencies onto a CCD. The CCD basically ”counts” the photons according to wavelength. The data is transferred via a usb port to a PC. The Ocean Optics software displays the spectrum as counts versus wavelength. We will examine the spectra given off by the following sources: incandescent light filament, hydrogen, helium, various light emitting diodes and a laser pointer. You will also be given an unknown gas discharge tube and will need to identify the gas via its spectral emissions Pre Lab 1) Obtain a spectrum for hydrogen, mercury, sodium and neon gas emissions. The spectrum must contain an accurate listing of major emission lines (in nm), not simply a color photo of the emission. 2) Make yourself familiar with the manual for the spectrometer and the software. These are available via the following links: http://www.oceanoptics.com/technical/hr4000.pdf http://www.oceanoptics.com/technical/SpectraSuite.pdf These documents are also available on Black Board. 3) How does the Ocean Optics spectrometer work? In your answer list its three main components and describe what each component does. -

Portable Fluid Analysis Kit Manual
Donaldson Delivers Portable Fluid Analysis Kit Patch Test Kit Manual Kit Part Number X009329 Carrying Case Membrane Holder & Funnel Assembly P567863 Filter for Solvent Dispensing Bottle P567860 (Qty. 3) 500 ml Solvent Sampling Dispensing Bottle Pump P176431 Microscope 120 ml P567864 Sample Bottles (Qty. 6) Plastic Tubing P567861 (5 ft.) 1.2 micron Patch Covers Membrane P567912 (Qty. 150) Filters P567869 Zip Drive of (Qty. 100) Reference Information Membrane Filter Forceps Sharpie Analysis Cards (3”x5”) P567865 (Qty. 50) 5 micron Membrane Filters Marker P567868 (Qty. 50) Case Size: Height: 14.5”/368.3mm | Width: 19.25”/489mm | Depth: 7.75”/197mm | Case Weight: 9.95 lbs./4.51 kg 1 2 3 Assemble waste bottle, funnel- Install solvent* dispensing tube and Rinse the funnel-patch assembly patch assembly, and vacuum pump install solvent filter on end of the with the filtered solvent to remove to form the sample processing dispensing tube. background contamination. The assembly. Tighten the vacuum patch should not be in place for pump o-ring on the funnel-patch * Mineral spirits are the most this process. assembly tube by turning the commonly used solvent aluminum locking device. 2 • www.donaldson.com Patch Test Kit Manual 4 5 6 Separate the funnel from the patch Reattach the funnel to the filter Agitate the sample fluid bottle and supporter and install a filter patch patch base with filter patch. Twist pour 25ml into the funnel. 25ml with ink grid up. (If the patch has an lock the funnel to the base. is denoted by the first line on the ink grid) funnel (closest to the patch). -

Marsh Funnel
Marsh Funnel Summary: The Marsh Funnel was developed to provide a field measurement of the apparent viscosity of clay-thickened drilling mud. Provided that a conversion table has been developed, a modified Marsh Funnel can be used to provide a measure of apparent viscosities for gum-thickened and polymer- thickened products used in wildland fire operations. Without a conversion table, the flow-through times can be used to determine batch to batch consistency of a single product, and in limited circumstances to compare similar products. The directions for use of a Marsh Funnel are given below. The procedures for modifying the Marsh Funnel for use in determining the viscosity of long-term retardants (Forest Service modification) and the consistency of water enhancers (California Department of Forestry and Fire Protection, CDF modification) are provided in the following pages. Materials: Marsh Funnel (Use small tip, 3/16” diameter, for low-viscosity products such as Phos-Chek 259 or Phos-Chek LC-95A. Use the large tip, 17/64” diameter, for high- viscosity products such as Phos-Chek D75.) Container marked to 1 quart, a tall straight-sided container is best Stopwatch or timer with a “time up” function Thermometer Method: 1. Allow the test sample to stand until it reaches room temperature (70°F) and all air bubbles are dissipated since both of these factors have an influence on viscosity. 2. Ensure that the properly modified funnel is clean and dry and secured in an upright position (a ring stand works fine). 3. Cover the funnel orifice with a finger while pouring the test sample through the screen until the sample exactly reaches the bottom of the screen. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout