Nose-Introduction-Pdf 508.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

EPITHELIAL TISSUE Or EPITHELIUM • the Basic Tissue of the Body
13.11.2014 Epithelium Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow EPITHELIAL TISSUE or EPITHELIUM • The basic tissue of the body. • Cells are arranged as continuous sheets. • Single or multiple layers. • Cells are held tightly together by cell junctions. • Free surface • Basal surface adheres to basal lamina or basement membrane. • Avascular but supplied by nerves. • Has high capability to regenerate. Embryological aspect • Epithelia are derived from all the 3 germ layers: • Ectoderm- Epithelium of skin • Endoderm- Epithelium of gut • Mesoderm- Epithelium of pericardial, peritoneal and pleural cavities Functions – Protection – Absorption – Barrier – Excretion – Secretory – Function as sensory surfaces Classification According to shape, arrangement and the specialization of their free surface: • Simple • Stratified • Pseudostratified • Transitional Simple epithelium Simple Squamous Epithelium • Single layered • Flat cells • On surface view, like floor tiles • Elevated nuclei Squamous • Examples: cell - Lung alveoli Nucleus - Parietal layer of Bowman’s capsule of kidney Basement - Inner aspect of membrane tympanic membrane Function: Rapid transport of - Mesothelium substances, secretion of fluid, - Endothelium diffusion of gases and osmosis Simple Squamous Epithelium Simple Cuboidal Epithelium • Single layer of cuboidal shaped cells • On surface view, cells look like mosaic (hexagonal) • Examples: -Thyroid follicles -Tubules of nephrons - Pigmented layer of retina - Germinal layer of ovary - Inner layer of -
Epithelial Tissue
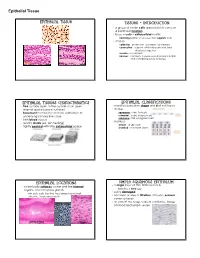
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Cell Replacement and Differentiation in Transitional Epithelium: a Histological and Autoradiographic Study of the Guinea-Pig Bladder and Ureter B
J. Anat. (1972), 112, 3, pp. 433-455 433 With 52 figures Printed in Great Britain Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter B. F. MARTIN Department of Anatomy, University of Birmingham (Accepted 9 June 1972) INTRODUCTION Transitional epithelium occupies a position between the pseudostratified and truly stratified epithelia, and there has been much conjecture regarding the arrangement of the constituent cells, their behaviour during stretching of the epithelium, and the manner in which they undergo replacement and differentiation. In a previous study on the guinea-pig (Martin, 1962) it was concluded that there are essentially three layers of cells in the epithelium, namely, a layer of small basal cells, a middle or intermediate layer of pyriform cells, which vary in size and height but retain connexion with the basement membrane by long thin cytoplasmic pro- cesses, and a layer of surface cells which are of large size and often bi- or multi- nucleate. During distension, the stretched epithelium of both bladder and ureter retains the essentially three-layer state; the surface cells become flattened and thus increase their surface area, whilst the underlying cells are not only flattened but are displaced sideways and may lie almost parallel to the basement membrane (Martin, 1962; Petry & Amon, 1966). In contrast with stratified squamous epithelia, in which there is a rapid rate of cell replacement from the basal layer, study of cell kinetics in transitional epithelium has proved difficult. Mitotic figures are seldom seen in the adult bladder and very few cells are labelled in autoradiographs following the administration of [3H]thymidine (3H-T). -

Histology of Compound Epithelium
Compound Epithelium Dr. Gitanjali Khorwal Learning objectives • Definition • Types • Function • Identification • Surface modifications of epithelia • SIMPLE • STRATIFIED One cell layer thick Two or more cell layer thick Squamous, Stratified Squamous, Stratified Cuboidal, Cuboidal, Stratified Columnar Pseudostratified Columnar Transitional Cell polarity refers to spatial differences in shape, structure, and function within a cell. • Apical domain • Lateral domain • Basal domain Apical domain modifications • Microvilli • Stereocilia/ Stereovilli • Cilia Microvilli • Finger-like cytoplasmic projections on the apical surface (1-3 micron). • Striated border- regular arrangement • Brush border - irregular arrangement Stereocilia/ Stereovilli • Unusually long, (120 micron) • immotile microvilli. • Epididymis, ductus deferens, Sensory hair cell of inner ear. Cilia • Hairlike extensions of apical plasma membrane • Contain axoneme-microtubule based internal structure. 1. Motile 9+2 2. Primary 9+0 3. Nodal : embryonic disc during gastrulation Stratified squamous epithelium (Non-keratinised) • Variable cell layers-thickness • The deepest cells - basal cell layer are cuboidal or columnar in shape. • mitotically active and replace the cells of the epithelium • layers of cells with polyhedral outlines. • Flattened surface cells. Stratified squamous epithelium (Keratinised) Stratified cuboidal epithelium • A two-or more layered cuboidal epithelium • seen in the ducts of the sweat glands, pancreas, salivary glands. Stratified columnar epithelium • excretory -

Use of Antibodies to Carcinoembryonic Antigen and Human Milk Fat Globule to Distinguish Carcinoma, Mesothelioma, and Reactive Mesothelium
J Clin Pathol 1984;37:1215-1221 Use of antibodies to carcinoembryonic antigen and human milk fat globule to distinguish carcinoma, mesothelioma, and reactive mesothelium RJ MARSHALL, A HERBERT, SG BRAYE, DB JONES From the University Department ofHistopathology, Southampton General Hospital, Southampton SUMMARY Antibodies raised against human milk fat globule (HMFG 1 and 2) and carcinoem- bryonic antigen were used in an immunoperoxidase technique to differentiate mesothelioma, carcinoma, and benign, reactive mesothelium. Sixteen mesotheliomas, 27 lung carcinomas, and 13 specimens of reactive mesothelium were examined. Staining for carcinoembryonic antigen was not seen in reactive mesothelium or mesothelioma but was present in 22 of 27 carcinomas. Mesothelioma and carcinoma usually stained with HMFG 1 and 2; reactive mesothelium did not. These three antibodies may help to distinguish carcinoma, mesothelioma, and reactive mesothelium. Distinguishing mesothelioma from carcinoma is a malignant mesothelioma was obtained from well recognised problem.' Histochemistry and elec- pleuro-pneumonectomy and pleurectomy specimens tron microscopy may help to make this distinction or from biopsies taken at thoracoscopy or but do not always give a definitive answer. Anti- thoracotomy (Table 1). Adequate material was bodies to carcinoembryonic antigen (CEA) and available in all cases for definitive diagnosis to be keratin have been assessed with conflicting made of biphasic (seven cases), epithelial (eight results.) It is equally difficult to distinguish benign cases), or mesenchymal (one case) malignant from malignant mesothelium. Morphometry6 and mesothelioma using morphological criteria. All the use of histiocytic markers in an immunoperoxid- cases were stained with periodic acid Schiff after ase technique8 have been advocated for this pur- diastase digestion and were negative. -

Microscopic Anatomy of the Lower Respiratory System of the African Giant Pouched Rat (Cricetomys Gambianus, Waterhouse 1840)
Int. J. Morphol., 29(1):27-33, 2011. Microscopic Anatomy of the Lower Respiratory System of the African Giant Pouched Rat (Cricetomys gambianus, Waterhouse 1840) Anatomía Microscópica del Sistema Respiratorio Inferior de la Rata Gigante Africana (Cricetomys gambianus, Waterhouse 1840) C. S. Ibe; B. I. Onyeanusi; S. O. Salami & J. O. Nzalak IBE, C. S.; ONYEANUSI, B. I.; SALAMI, S. O. & NZALAK, J. O. Microscopic anatomyof the lower respiratory system of the African giant pouched rat (Cricetomys gambianus, Waterhouse 1840). Int. J. Morphol., 29(1):27-33, 2011. SUMMARY:A qualitative and quantitative study, by light microscopy, was undertaken on the lower respiratory system of the African Giant pouched rat. Specifically, the trachea, bronchi and lungs were stained with Haematoxylin and eosin, Alcian blue at a pH of 2.5 and Periodic Acid-Schiff stains. Three cell types were identified in saggital sections of the trachea: the ciliated cells, basal cells and mucous cells. Fibers of the trachealis muscles in the laminar propria separated the underlying cartilages from the basal cells. Mucous cells were visible only in the membranous portion of the trachea and they were predominant in the rostral and caudal portion of the trachea. Lobar bronchi consisted of cuboidal epithelium and a layer of one or two smooth muscle cells and opened into segmental bronchi and respiratory bronchiole. Some tracheal cartilaginous rims stained blue with AB while most glandular cells stained red with PAS. The diameter of respiratory bronchiole, alveoli duct and alveoli were 24.93 µm (± 1.27), 21.14 µm (± 0.66) and 12.95 µm (± 0.21), respectively. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

Squamous Epithelium Are Thin, Which Allows for the Rapid Passage of Substances Through Them
Chapter 2 1st Prof. Anatomy Arsalan (Lecturer Department of Pharmacy University of Peshawar) Tissue is an aggregation of similar cells and their products that perform same function. There are four principal types of tissues in the body: ❑ epithelial tissue: covers body surfaces, lines body cavities and ducts and forms glands ❑ connective tissue: binds, supports, and protects body parts ❑ muscle tissue: produce body and organ movements ❑ nervous tissue: initiates and transmits nerve impulses from one body part to another • Epithelial tissues cover body and organ surfaces, line body cavities and lumina and forms various glands • Derived from endoderm ,ectoderm, and mesoderm • composed of one or more layers of closely packed cells • Perform diverse functions of protection, absorption, excretion and secretion. Highly cellular with low extracellular matrix Polar – has an apical surface exposed to external environment or body cavity, basal layer attached to underlying connective tissue by basement membrane and lateral surfaces attached to each other by intercellular junctions Innervated Avascular – almost all epithelia are devoid of blood vessels, obtain nutrients by diffusion High regeneration capacity Protection: Selective permeability: in GIT facilitate absorption, in kidney facilitate filtration, in lungs facilitate diffusion. Secretions: glandular epithelium form linings of various glands, involved in secretions. Sensations: contain some nerve endings to detect changes in the external environment at their surface Epithelium rests on connective tissue. Between the epithelium and connective tissue is present the basement membrane which is extracellular matrix made up of protein fibers and carbohydrates. Basement membrane attach epithelium to connective tissue and also regulate movement of material between epithelium and connective tissue Epithelial cells are bound together by specialized connections in the plasma membranes called intercellular junctions . -

HISTOLOGY DRAWINGS Created by Dr Carol Lazer During the Period 2000-2005
HISTOLOGY DRAWINGS created by Dr Carol Lazer during the period 2000-2005 INTRODUCTION The first pages illustrate introductory concepts for those new to microscopy as well as definitions of commonly used histology terms. The drawings of histology images were originally designed to complement the histology component of the first year Medical course run prior to 2004. They are sketches from selected slides used in class from the teaching slide set. These labelled diagrams should closely follow the current Science courses in histology, anatomy and embryology and complement the virtual microscopy used in the current Medical course. © Dr Carol Lazer, April 2005 STEREOLOGY: SLICING A 3-D OBJECT SIMPLE TUBE CROSS SECTION = TRANSVERSE SECTION (XS) (TS) OBLIQUE SECTION 3-D LONGITUDINAL SECTION (LS) 2-D BENDING AND BRANCHING TUBE branch off a tube 2 sections from 2 tubes cut at different angles section at the beginning 3-D 2-D of a branch 3 sections from one tube 1 section and the grazed wall of a tube en face view = as seen from above COMPLEX STRUCTURE (gland) COMPOUND ( = branched ducts) ACINAR ( = bunches of secretory cells) GLAND duct (XS =TS) acinus (cluster of cells) (TS) duct and acinus (LS) 3-D 2-D Do microscope images of 2-D slices represent a single plane of section of a 3-D structure? Do all microscope slides show 2-D slices of 3-D structures? No, 2-D slices have a thickness which can vary from a sliver of one cell to several cells deep. No, slides can also be smears, where entire cells With the limited depth of field of high power lenses lie on the surface of the slide, or whole tissue it is possible to focus through the various levels mounts of very thin structures, such as mesentery. -

EPIDERMIZATION, OF, the TRANSITIONAL EPITHELIUM LINING the PELVIS of the KIDNEY·, FOLLOWED by SQUAMOUS-CELLED 0ARCINOMA,AND OT~ER CHANGES'~ by MAJOR J
J R Army Med Corps: first published as 10.1136/jramc-41-05-05 on 1 November 1923. Downloaded from \ ' 365 , ' EPIDERMIZATION, OF, THE TRANSITIONAL EPITHELIUM LINING THE PELVIS OF THE KIDNEY·, FOLLOWED BY SQUAMOUS-CELLED 0ARCINOMA,AND OT~ER CHANGES'~ By MAJOR J. A. MANIFOLD, D.S.O. , Royal Army Medical Corp8. THE following notes of a ~ew growth ,of the k1dney are thought' to be of sufficient interest to b,e placed pn record, as: some· unusual pathological. 'features were noted in the neoplasQI. The' kidney was removed from a patient aged 52, who had suffered from chronic pyonephrosis: When the organ was prepared for microscopic slab sec.tions there was found, in addition to the pyonephrosis, a growth of \ new tissue apparently originating in the pelvis of the kidney, which· called for further investigation. ' ' guest. Protected by copyright. , MACROSCOPIC ApPEARANCE. The small portion of, unaltered renal tissue which remained almost encapsuled a growth about the size of a small orange situated in the renal pelvis. In its substance'a few !,cattered cysts were evident. Some of . these cysts were lined with. a layer of vvhite tissue and th~ir contents were flesh coloured, in others, only parti~lly so lined, the 90ntents were greenish in colour (fig. 1). - MICROSCOFIC ApPEARANCE. It was' found that the normal transitional lining epith~lium ~f the 'pelvis of the kidney had undergone complete metamoFphosjs, having taRen tlie character of !,urface e'IJitheliuni; the stratified layer being well formed; 'and extensillely keratinized. ' " ' . , Portions of' this stratified epithelium infiltrated the, kidney substance. http://militaryhealth.bmj.com/ The cells in the centre of the infiltrating columns became clear,~ swollen and finally keratinized, thus.