Rifaximin (Xifaxan®) PAGE: 1 of 5 REFERENCE NUMBER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibiotic Treatment in Inflammatory Bowel Disease: Rifaximin, a New Possible Approach
European Review for Medical and Pharmacological Sciences 1999; 3: 27-30 Review – Antibiotic treatment in inflammatory bowel disease: rifaximin, a new possible approach P. GIONCHETTI, F. RIZZELLO, A. VENTURI, F. UGOLINI*, M. ROSSI**, P. BRIGIDI**, R. JOHANSSON, A. FERRIERI***, G. POGGIOLI*, M. CAMPIERI Clinica Medica I, Nuove Patologie, Bologna University (Italy) *Istituto di Clinica Chirurgica II, Bologna University (Italy) **Dipartimento di Scienze Farmaceutiche, Bologna University (Italy) ***Direzione Medica Alfa Wassermann, Bologna (Italy) Abstract. – The etiology of inflammatory ported the presence of a breakdown of toler- disease is still unknown, but a body of evidence ance to the normal commensal intestinal flora from clinical and experimental observation indi- in active IBD2-3. Reduction of microflora by cates a role for intestinal microflora in the pathogenesis of this disease. Reduction of mi- antibiotics, bowel rest and fecal diversion de- croflora using antibiotics, bowel rest and fecal creases inflammatory activity in Crohn’s dis- diversion decreases activity in Crohn’s disease ease and to a lesser extent in ulcerative colitis and in ulcerative colitis. Several trials have been (UC). The pathogenetic role of intestinal mi- carried out on the use of antibiotic treatment in croflora is further suggested by the observa- patients with active ulcerative colitis with con- tion that experimental colitis, in germ-free or trasting results. A number of trials have been antibiotic treated animals, is less severe com- carried out using Rifaximin, a non-absorbable broad-spectrum antibiotic, confirming the ab- pared to that inducible in normal animals or sence of systemic bioavalaibility of the drug at times is not inducible at all. -

FDA Warns About an Increased Risk of Serious Pancreatitis with Irritable Bowel Drug Viberzi (Eluxadoline) in Patients Without a Gallbladder
FDA warns about an increased risk of serious pancreatitis with irritable bowel drug Viberzi (eluxadoline) in patients without a gallbladder Safety Announcement [03-15-2017] The U.S. Food and Drug Administration (FDA) is warning that Viberzi (eluxadoline), a medicine used to treat irritable bowel syndrome with diarrhea (IBS-D), should not be used in patients who do not have a gallbladder. An FDA review found these patients have an increased risk of developing serious pancreatitis that could result in hospitalization or death. Pancreatitis may be caused by spasm of a certain digestive system muscle in the small intestine. As a result, we are working with the Viberzi manufacturer, Allergan, to address these safety concerns. Patients should talk to your health care professional about how to control your symptoms of irritable bowel syndrome with diarrhea (IBS-D), particularly if you do not have a gallbladder. The gallbladder is an organ that stores bile, one of the body’s digestive juices that helps in the digestion of fat. Stop taking Viberzi right away and get emergency medical care if you develop new or worsening stomach-area or abdomen pain, or pain in the upper right side of your stomach-area or abdomen that may move to your back or shoulder. This pain may occur with nausea and vomiting. These may be symptoms of pancreatitis, an inflammation of the pancreas, an organ important in digestion; or spasm of the sphincter of Oddi, a muscular valve in the small intestine that controls the flow of digestive juices to the gut. Health care professionals should not prescribe Viberzi in patients who do not have a gallbladder and should consider alternative treatment options in these patients. -

Formulary Additions
JUNE 2016 FORMULARY ADDITIONS MELOXICAM (MOBIC®) Rationale for Addition: Meloxicam is a once daily NSAID used for the treatment of rheumatoid arthritis and osteoarthritis. Meloxicam will provide another oral NSAID option to Celecoxib for patients who are being discharged home and whose insurance does not cover celecoxib. Adverse Effects: abdominal pain, diarrhea, dyspepsia, flatulence, nausea, edema, influenza-like symptoms, dizziness, headache, rash Contraindications: peri-operative pain in setting of coronary artery bypass graft surgery Drug Interactions: other NSAIDS, antiplatelet agents, calcium polysterene sulfonate, cyclosporine, digoxin, lithium, methotrexate, vancomycin, vitamin K antagonist, corticosteroids Dosage & Administration: meloxicam 7.5 mg PO daily, some patients my experience some benefit by increasing to 15 mg PO daily Renal Impairment: not recommended in patients with renal function ≤ 20 mL/min Dosage Form & Cost: meloxicam 7.5 mg tablet $0.02/day meloxicam 15 mg $0.03/day IVABRADINE (CORLANOR®) *Restrictions*: restricted to cardiology only for initiation of therapy Rationale for Addition: Ivabradine blocks the funny current leading to a reduction in heart rate at the sinoatrial node. Ivabradine is indicated to reduce the risk of hospitalization for worsening heart failure in patients with stable symptomatic chronic heart failure with LVEF ≤ 35% who are in sinus rhythm with resting heart rate (HR) ≥ 70 beats per minute and either on maximally tolerated doses of β-blockers or have a contraindication to β-blockers. -

Erythromycin Versus Tetracycline for Treatment of Mediterranean Spotted Fever
Arch Dis Child: first published as 10.1136/adc.61.10.1027 on 1 October 1986. Downloaded from Archives of Disease in Childhood, 1986, 61, 1027-1029 Erythromycin versus tetracycline for treatment of Mediterranean spotted fever T MUNOZ-ESPIN, P LOPEZ-PARtS, E ESPEJO-ARENAS, B FONT-CREUS, I MARTINEZ- VILA, J TRAVERIA-CASANOVA, F SEGURA-PORTA, AND F BELLA-CUETO Hospital de Sant Llatzer, Terrassa, Clinica Infantil del Nen Jesus, Sabadell, and Hospital Mare de Deu de la Salut, Sabadell, Barcelona, Spain SUMMARY Eighty one children aged between 1 and 13 years participated in a randomised comparative trial of tetracycline hydrochloride and erythromycin stearate for treatment of Mediterranean spotted fever. Both therapeutic regimens proved effective, but in patients treated with tetracycline both clinical symptoms and fever disappeared significantly more quickly. Likewise, when those patients who began treatment within the first 72 hours of illness are considered the febrile period had a significantly shorter duration in the group treated with tetracycline. One patient was switched to tetracycline because there was no improvement of clinical manifestations, with persistence of fever, myalgias, and prostration, after receiving eight days of treatment with erythromycin. These results suggest that tetracyclines are superior to erythromycin in the treatment of Mediterranean spotted fever. copyright. Mediterranean spotted fever is an acute infectious were not included in the trial; neither were those disease caused by Rickettsia conorii. During the -

Non-Hepatic Hyperammonaemia: an Important, Potentially Reversible Cause of Encephalopathy
Postgrad Med J 2001;77:717–722 717 Postgrad Med J: first published as 10.1136/pmj.77.913.717 on 1 November 2001. Downloaded from CASE REPORTS Non-hepatic hyperammonaemia: an important, potentially reversible cause of encephalopathy N D Hawkes, G A O Thomas, A Jurewicz, O M Williams, C E M Hillier, I N F McQueen, G Shortland Abstract Case reports The clinical syndrome of encephalopathy CASE 1 is most often encountered in the context of A 20 year old man was admitted to a local hos- decompensated liver disease and the diag- pital with two days of inappropriate behaviour, nosis is usually clear cut. Non-hepatic clumsiness, drowsiness, memory loss, slurred causes of encephalopathy are rarer and speech, and abdominal discomfort. Since the tend to present to a wide range of medical age of 2 years he had suVered recurrent rectal specialties with variable and episodic bleeding and investigation had revealed haem- symptoms. Delay can result in the devel- orrhoids. Bleeding from his rectum had contin- opment of potentially life threatening ued over the years but had been worse recently. complications, such as seizures and coma. On examination he was confused. His Early recognition is vital. A history of Glasgow coma scale score (GCS) was 15/15. similar episodes or clinical risk factors Neurological and general examination was nor- and early assessment of blood ammonia mal, with no stigmata of chronic liver disease. levels help establish the diagnosis. In Investigations showed a leucocytosis (leuco- × 9 addition to adequate supportive care, cyte count 22 10 /l) and serum bilirubin level investigation of the underlying cause of of 32 µmol/l. -

Use of Antibiotics to Prevent Calf Diarrhea and Septiceinia
PEER REVIEWED Use of Antibiotics to Prevent Calf Diarrhea and Septiceinia Peter D. Constable, BVSc, PhD, DACVIM Department of Veterinary Clinical Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802 Abstract l'oxytetracycline (0.15 to 6.0 mg/lb [0.32 to 13.2 mg/kg] au 24 h, per os) sont efficaces dans le traitement de la This article reviews studies related to the use of diarrhee chez les veaux et permettent un accroissement antimicrobial agents to prevent calf diarrhea and septi du taux de croissance des veaux nourris au lait. De cemia, and discusses whether prophylactic administra plus, la chlortetracycline permet la diminution du taux tion of antibiotics in neonatal calves is effective and de mortalite lorsque administree chez les veaux indicated. Orally administered chlortetracycline, oxytet nouveau-nes pour prevenir la diarrhee. 11 n'y a pas racycline, tetracycline and neomycin have label claims d'antibiotiques qui promettent de prevenir la in the US for the "control" or "aid in the control" of calf septicemie chez les veaux. Paree qu'il ne semble pas diarrhea caused by bacteria susceptible to the antibi exister d'etudes sur l'efficacite de la tetracycline et de otic. Chlortetracycline and oxytetracycline (0.15 to 6.0 la neomycine et parce que !'utilisation hors homologa mg/lb [0.32 to 13.2 mg/kg], q 24 h, PO) are efficacious tion des medicaments. n'est pas permise pour la for preventing calf diarrhea and increasing growth rate prevention routiniere des maladies ou !'augmentation in milk-fed calves, and chlortetracycline (3 mg/lb [7 mg/ du taux de croissance et du taux de conversion kg], q 12 h, PO) is efficacious for decreasing mortality alimentaire, selon le code de l'AMDUCA de 1994, seule when administered to prevent diarrhea in neonatal !'administration orale de chlortetracycline et calves. -

Picquestion of the Week:3/09/09
McKechnie Field - Spring Training Home of the Pirates PIC QUESTION OF THE WEEK: 3/09/09 Q: Why is rifaximin used in patients with pouchitis? A: Pouchitis is an inflammation of the internal pouch fashioned from small intestinal tissue during ileal pouch anal anastamosis (IPAA). This surgical procedure bypasses the large intestine and may be employed in patients with ulcerative colitis or Crohn’s disease. A temporary ileostomy is placed to allow the pouch to heal without risk of infection. IPAA is considered preferable to an ostomy for patients suffering from inflammatory bowel diseases refractory to medical treatment. The pouch serves as a collection device for waste, but permits the patient to experience regular bowel movements. It is typical for patients with an internal pouch to experience more frequent (average 6 per day) and watery bowel movements. Pouchitis is the most common complication of IPAA and widely regarded as an idiopathic disease; however, colonization by fecal bacteria may be a contributing factor. The condition occurs most commonly in the first six months following reversal of the ileostomy. Although its frequency decreases after six months, nearly 50% of patients with an IPAA will eventually experience pouchitis. Presenting symptoms include diarrhea, increased frequency of bowel movements, bleeding, abdominal pain, and fever. It can result in dehydration and, in severe cases, hospitalization. Treatment of acute pouchitis generally consists of a two-week course of antibiotic therapy with metronidazole or ciprofloxacin. Approximately 10% of patients do not respond to initial treatment and develop chronic (> 4 weeks) pouchitis. A small number of clinical trials support the potential use of rifaximin for the treatment of refractory or recurrent pouchitis. -

Long-Term Use of Spironolactone for Acne in Women: a Case Series of 403 Patients
Long-term use of spironolactone for acne in women: A case series of 403 patients Vaibhav Garg, BS,a JulianaK.Choi,MD,PhD,b William D. James, MD,b and John S. Barbieri, MD, MBAb Philadelphia, Pennsylvania Background: There are limited data regarding the long-term outcomes of spironolactone use for women with acne and its effect on truncal acne. Objective: To comprehensively describe outcomes of patients treated with spironolactone in routine clinical practice, including long-term outcomes. Methods: We performed a retrospective case series of 403 adult women treated for acne with spironolactone at an academic medical center between 2008 and 2019. Rates of objective, as assessed by Comprehensive Acne Severity Scale scores, and subjective acne clearance were evaluated, as well as rates of treatment discontinuation, dosage changes, and drug survival. Logistic regression was used to assess for association between incidence of menstrual adverse effects and combined oral contraceptive use. Results: As evaluated by Comprehensive Acne Severity Scale scores, at the first follow-up, 75.5%, 84.0%, and 80.2% of patients with available data had reduction or complete clearance of acne on the face, chest, and back, respectively. The mean drug survival was 470.7 days. Menstrual adverse effects were less common among those using combined oral contraception (odds ratio, 0.23; 95% confidence interval, 0.11-0.50). Limitations: This study was conducted at a single academic medical center. Conclusions: Spironolactone improves clinical outcomes and is well tolerated for many adult women with acne using it for an extended duration. ( J Am Acad Dermatol 2021;84:1348-55.) Key words: acne; acne vulgaris; birth control pill; combined oral contraceptive; comprehensive acne severity scale; oral antibiotics; outcomes; spironolactone. -

Prednisolone / Neomycin / Tetracycline For- Mulation
SAFETY DATA SHEET Prednisolone / Neomycin / Tetracycline For- mulation Version Revision Date: SDS Number: Date of last issue: 25.08.2020 3.6 09.04.2021 407521-00015 Date of first issue: 07.01.2016 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Trade name : Prednisolone / Neomycin / Tetracycline Formulation 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the Sub- : Veterinary product stance/Mixture 1.3 Details of the supplier of the safety data sheet Company : MSD 20 Spartan Road 1619 Spartan, South Africa Telephone : +27119239300 E-mail address of person : [email protected] responsible for the SDS 1.4 Emergency telephone number +1-908-423-6000 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification (REGULATION (EC) No 1272/2008) Skin sensitisation, Category 1 H317: May cause an allergic skin reaction. Reproductive toxicity, Category 1A H360D: May damage the unborn child. Effects on or via lactation H362: May cause harm to breast-fed children. Short-term (acute) aquatic hazard, Cate- H400: Very toxic to aquatic life. gory 1 Long-term (chronic) aquatic hazard, Cat- H410: Very toxic to aquatic life with long lasting egory 1 effects. 2.2 Label elements Labelling (REGULATION (EC) No 1272/2008) Hazard pictograms : Signal word : Danger Hazard statements : H317 May cause an allergic skin reaction. H360D May damage the unborn child. H362 May cause harm to breast-fed children. H410 Very toxic to aquatic life with long lasting effects. 1 / 23 SAFETY DATA SHEET Prednisolone / Neomycin / Tetracycline For- mulation Version Revision Date: SDS Number: Date of last issue: 25.08.2020 3.6 09.04.2021 407521-00015 Date of first issue: 07.01.2016 Precautionary statements : Prevention: P201 Obtain special instructions before use. -

Diagnosis and Management of Primary Biliary Cholangitis Ticle
REVIEW ArtICLE 1 see related editorial on page x Diagnosis and Management of Primary Biliary Cholangitis TICLE R Zobair M. Younossi, MD, MPH, FACG, AGAF, FAASLD1, David Bernstein, MD, FAASLD, FACG, AGAF, FACP2, Mitchell L. Shifman, MD3, Paul Kwo, MD4, W. Ray Kim, MD5, Kris V. Kowdley, MD6 and Ira M. Jacobson, MD7 Primary biliary cholangitis (PBC) is a chronic, cholestatic, autoimmune disease with a variable progressive course. PBC can cause debilitating symptoms including fatigue and pruritus and, if left untreated, is associated with a high risk of cirrhosis and related complications, liver failure, and death. Recent changes to the PBC landscape include a REVIEW A name change, updated guidelines for diagnosis and treatment as well as new treatment options that have recently become available. Practicing clinicians face many unanswered questions when managing PBC. To assist these healthcare providers in managing patients with PBC, the American College of Gastroenterology (ACG) Institute for Clinical Research & Education, in collaboration with the Chronic Liver Disease Foundation (CLDF), organized a panel of experts to evaluate and summarize the most current and relevant peer-reviewed literature regarding PBC. This, combined with the extensive experience and clinical expertise of this expert panel, led to the formation of this clinical guidance on the diagnosis and management of PBC. Am J Gastroenterol https://doi.org/10.1038/s41395-018-0390-3 INTRODUCTION addition, diagnosis and treatment guidelines are changing and a Primary biliary cholangitis (PBC) is a chronic, cholestatic, auto- number of guidelines have been updated [4, 5]. immune disease with a progressive course that may extend over Because of important changes in the PBC landscape, and a num- many decades. -

Profiling the Effects of Rifaximin on the Healthy Human Colonic Microbiota Using A
bioRxiv preprint doi: https://doi.org/10.1101/828269; this version posted November 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Profiling the effects of rifaximin on the healthy human colonic microbiota using a 2 chemostat model 3 4 Ines B. Mouraa#, Anthony M. Buckleya, Duncan Ewina, Emma Clarka, Suparna Mitraa, Mark 5 H. Wilcoxa,b, Caroline H. Chiltona 6 7 aLeeds Institute of Medical Research, Faculty of Medicine and Health, University of Leeds, 8 Leeds, UK; 9 bDepartment of Microbiology, Leeds Teaching Hospitals NHS Trust, The General Infirmary, 10 Leeds, UK 11 12 Keywords: Rifaximin, gut microbiota, chemostat model, 16S rRNA sequencing 13 14 # Corresponding Author: Dr Ines Moura 15 Healthcare Associated Infection Research Group 16 Leeds Institute of Medical Research 17 University of Leeds 18 Microbiology, Old Medical School 19 Leeds General Infirmary 20 Leeds LS1 3EX, UK 21 Tel +44 113 392 8663 22 Email: [email protected] 23 24 25 bioRxiv preprint doi: https://doi.org/10.1101/828269; this version posted November 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 2 26 Abstract 27 28 Rifaximin is a low solubility antibiotic with activity against a wide range of bacterial 29 pathogens. -
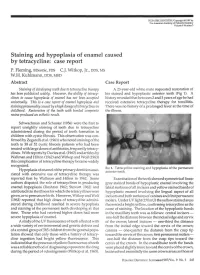
Staining and Hypoplasia of Enamel Caused by Tetracycline: Case Report P
PEDIATRIC DENTISTRY/Copyright © 1987 by The American Academy of Pediatric Dentistry Volume 9 Number 3 Staining and hypoplasia of enamel caused by tetracycline: case report P. Fleming, BDentSc, FDS C.J. Witkop, Jr., DDS, MS W.H. Kuhlmann, DDS, MSD Abstract Case Report Staining of developing teeth due to tetracycline therapy A 23-year-old white male requested restoration of has been publicized widely. However, the ability of tetracy- his stained and hypoplastic anterior teeth (Fig 1). A clines to cause hypoplasia of enamel has not been accepted history revealed that between 2 and 3 years of age he had universally. This is a case report of enamel hypoplasia and received extensive tetracycline therapy for tonsillitis. staining presumably caused byahigh dosage of'tetracy'dines in There was no history of a prolonged fever at the time of childhood. Restoration of the teeth with bonded composite the illness. resins produced an esthetic result. -UK Schwachman and Schuster (1956) were the first to report unsightly staining of teeth due to tetracycline administered during the period of tooth formation in children with cystic fibrosis. This observation was con- y firmed by Zegarelli et al. (1961) who noted staining of the teeth in 38 of 52 cystic fibrosis patients who had been ijgj treated with large doses of antibiotics, frequently tetracy- .^••W^^ clines. With reports by Davies et al. (1962) and articles by Wallman and Hilton (1962) and Witkop and Wolf (1963) this complication of tetracycline therapy became widely recognized. FIG 1. Tetracycline staining and hypoplasia of the permanent Hypoplasia of enamel of the primary dentition asso- anterior teeth.