Prednisolone / Neomycin / Tetracycline For- Mulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Erythromycin Versus Tetracycline for Treatment of Mediterranean Spotted Fever
Arch Dis Child: first published as 10.1136/adc.61.10.1027 on 1 October 1986. Downloaded from Archives of Disease in Childhood, 1986, 61, 1027-1029 Erythromycin versus tetracycline for treatment of Mediterranean spotted fever T MUNOZ-ESPIN, P LOPEZ-PARtS, E ESPEJO-ARENAS, B FONT-CREUS, I MARTINEZ- VILA, J TRAVERIA-CASANOVA, F SEGURA-PORTA, AND F BELLA-CUETO Hospital de Sant Llatzer, Terrassa, Clinica Infantil del Nen Jesus, Sabadell, and Hospital Mare de Deu de la Salut, Sabadell, Barcelona, Spain SUMMARY Eighty one children aged between 1 and 13 years participated in a randomised comparative trial of tetracycline hydrochloride and erythromycin stearate for treatment of Mediterranean spotted fever. Both therapeutic regimens proved effective, but in patients treated with tetracycline both clinical symptoms and fever disappeared significantly more quickly. Likewise, when those patients who began treatment within the first 72 hours of illness are considered the febrile period had a significantly shorter duration in the group treated with tetracycline. One patient was switched to tetracycline because there was no improvement of clinical manifestations, with persistence of fever, myalgias, and prostration, after receiving eight days of treatment with erythromycin. These results suggest that tetracyclines are superior to erythromycin in the treatment of Mediterranean spotted fever. copyright. Mediterranean spotted fever is an acute infectious were not included in the trial; neither were those disease caused by Rickettsia conorii. During the -

SDS: Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP SAFETY DATA SHEET
SDS: Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP SAFETY DATA SHEET 1. Identification Product Identifier: Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP Synonyms: Bacitracins, zinc complex, Neomycin B Sulfates, Polymyxin B Sulfates. National Drug Code (NDC): 17478-235-35 Recommended Use: Pharmaceutical. Company: Akorn, Inc. 1925 West Field Court, Suite 300 Lake Forest, Illinois 60045 Contact Telephone: 1-800-932-5676 E mail: [email protected] Emergency Phone Number: CHEMTREC 1-800-424-9300 (U.S. and Canada) 2. Hazard(s) Identification Physical Hazards: Not classifiable. Health Hazards: Not classifiable. Symbol(s): None. Signal Word: None. Hazard Statement(s): None. Precautionary Statement(s): None. Hazards Not Otherwise Classified: Not classifiable. Supplementary Information: While this material is not classifiable as hazardous under the OSHA standard, this SDS contains valuable information critical to safe handling and proper use of the product. This SDS should be retained and available for employees and other users of this product. 3. Composition/Information on Ingredients Chemical Name CAS Synonyms Chemical Formula Molecular Percentage Number Weight Neomycin Sulfate 1405-10-3 Neomycin B C23H46N6O13•3H2SO4 908.89 0.35% Sulfate Polymyxin B Sulfate 1405-20-5 Polymyxin B C43H82N16O12•xH2O4S 1701.97 10,000 Units Sulfate of Polymyxin B * The formula also contains Bacitracin Zinc equal to 400 units of Bacitracin units, and White Petrolatum. 1 of 8 SDS: Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, USP 4. First Aid Measures Ingestion: May cause irritation and hypersensitivity in some individuals. Ingestion of large quantities may induce gastric disturbances. -

Neomycin, Polymyxin B, and Dexamethasone
PATIENT & CAREGIVER EDUCATION Neomycin, Polymyxin B, and Dexamethasone This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Maxitrol Brand Names: Canada Dioptrol; Maxitrol What is this drug used for? It is used to treat or prevent eye infections. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. If your child has any of these health problems: A fungal infection, TB (tuberculosis), or viral infection of the eye. This is not a list of all drugs or health problems that interact with this drug. Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make Neomycin, Polymyxin B, and Dexamethasone 1/6 sure that it is safe to give this drug with all of your child’s other drugs and health problems. Do not start, stop, or change the dose of any drug your child takes without checking with the doctor. What are some things I need to know or do while my child takes this drug? Tell all of your child’s health care providers that your child is taking this drug. This includes your child’s doctors, nurses, pharmacists, and dentists. -

Use of Antibiotics to Prevent Calf Diarrhea and Septiceinia
PEER REVIEWED Use of Antibiotics to Prevent Calf Diarrhea and Septiceinia Peter D. Constable, BVSc, PhD, DACVIM Department of Veterinary Clinical Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802 Abstract l'oxytetracycline (0.15 to 6.0 mg/lb [0.32 to 13.2 mg/kg] au 24 h, per os) sont efficaces dans le traitement de la This article reviews studies related to the use of diarrhee chez les veaux et permettent un accroissement antimicrobial agents to prevent calf diarrhea and septi du taux de croissance des veaux nourris au lait. De cemia, and discusses whether prophylactic administra plus, la chlortetracycline permet la diminution du taux tion of antibiotics in neonatal calves is effective and de mortalite lorsque administree chez les veaux indicated. Orally administered chlortetracycline, oxytet nouveau-nes pour prevenir la diarrhee. 11 n'y a pas racycline, tetracycline and neomycin have label claims d'antibiotiques qui promettent de prevenir la in the US for the "control" or "aid in the control" of calf septicemie chez les veaux. Paree qu'il ne semble pas diarrhea caused by bacteria susceptible to the antibi exister d'etudes sur l'efficacite de la tetracycline et de otic. Chlortetracycline and oxytetracycline (0.15 to 6.0 la neomycine et parce que !'utilisation hors homologa mg/lb [0.32 to 13.2 mg/kg], q 24 h, PO) are efficacious tion des medicaments. n'est pas permise pour la for preventing calf diarrhea and increasing growth rate prevention routiniere des maladies ou !'augmentation in milk-fed calves, and chlortetracycline (3 mg/lb [7 mg/ du taux de croissance et du taux de conversion kg], q 12 h, PO) is efficacious for decreasing mortality alimentaire, selon le code de l'AMDUCA de 1994, seule when administered to prevent diarrhea in neonatal !'administration orale de chlortetracycline et calves. -

Comparison of Thimerosal Effectiveness in the Formulation of Eye Drops Containing Neomycin Sulfate and Chloramphenicol
International Journal of Applied Pharmaceutics ISSN- 0975-7058 Vol 11, Issue 1, 2019 Original Article COMPARISON OF THIMEROSAL EFFECTIVENESS IN THE FORMULATION OF EYE DROPS CONTAINING NEOMYCIN SULFATE AND CHLORAMPHENICOL MARLINE ABDASSAH1, SRI AGUNG FITRI KUSUMA2* 1Departement of Pharmaceutics, Faculty of Pharmacy, Padjadjaran University, Sumedang, West Java, Indonesia 45363, 2Department of Biology Pharmacy, Faculty of Pharmacy, Padjadjaran University, Sumedang, West Java, Indonesia 45363 Email: [email protected] Received: 27 Sep 2018, Revised and Accepted: 19 Nov 2018 ABSTRACT Objective: This study was aimed to compare the preservative efficacy of thimerosal in eye drops formulation containing neomycin sulfate and chloramphenicol as the active agents. Methods: Determination of thimerosal concentration in combinations with chloramphenicol and neomycin sulfate was carried out using the agar diffusion method. Then the thimerosal ineffective and minimal concentration was formulated into eye drops, each with 0.5% neomycin sulfate and 0.5% chloramphenicol as the active ingredient. Evaluation of eye drops was carried out for 28 d, which included: visual observation, pH measurement, sterility, and effectiveness test. Results: Thimerosal at a minimum concentration of 0.001% remain to provide antibacterial activity against common eyes contaminants. Both eyes drops containing neomycin sulfate, and chloramphenicol resulted in clear solution, sterile, and stable in the pH and antibacterial potency,showed the efficacy of thimerosal’s role in eye drops at the lowest concentration. But, the thimerosal stability as a preservative agent was affected by the pH values of the eye drops solution. Therefore, the effectivity of thimerosal in chloramphenicol (pH 7.19-7.22) was better than neomycin sulfate (6.45- 6.60). -

Conjunctivitis Or Worse?
Red Eye in Dogs and CatS: Conjunctivitis or Worse? Tracy Revoir, DVM Senior Manager of Veterinary Support, Dechra Veterinary Products It should come as no surprise that conjunctivitis is Common Causes of Conjunctivitis the most common ophthalmic disorder in dogs and cats. But because the clinical signs of conjunctivitis If you do confirm conjunctivitis, the next step is can mimic those of more serious ophthalmic identifying the cause. If both eyes are affected and diseases (glaucoma and uveitis), it’s important to abnormal clinical signs are apparent in other body confirm your diagnosis. systems, think underlying systemic disease. If only one eye is affected, rule out infection, tear film What are important clues to the severity of the deficiencies, an irritant, anatomical abnormality, condition? With conjunctivitis, the inflammation or deeper ocular disease. should be limited to the conjunctiva. Hyperemic conjunctival vessels are superficial, branching, In dogs, conjunctivitis can result from anatomical and bright red. They are movable over the deeper disorders, irritants, infection (usually bacterial), or episcleral vessels and can be blanched with topical atopy. Most bacterial infections are secondary dilute phenylephrine. With glaucoma and uveitis, the conditions, most often to allergies. In cats, herpes- episcleral vessels are engorged; they are dark red, virus and Chlamydophila felis are the most common deep, straight, and immobile and do not blanch with causes of conjunctivitis. Atopy can also be topical dilute phenylephrine. With conjunctivitis, an issue in cats. the Schirmer tear test and intraocular pressures are normal. And the cornea should be clear and no aqueous flare should be present. The pupil and Addressing the Problem pupillary responses are normal and intraocular structures should be visible. -

Topical Antibiotics for Impetigo: a Review of the Clinical Effectiveness and Guidelines
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Topical Antibiotics for Impetigo: A Review of the Clinical Effectiveness and Guidelines Service Line: Rapid Response Service Version: 1.0 Publication Date: February 21, 2017 Report Length: 23 Pages Authors: Rob Edge, Charlene Argáez Cite As: Topical antibiotics for impetigo: a review of the clinical effectiveness and guidelines. Ottawa: CADTH; 2017 Feb. (CADTH rapid response report: summary with critical appraisal). ISSN: 1922-8147 (online) Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. -

Long-Term Use of Spironolactone for Acne in Women: a Case Series of 403 Patients
Long-term use of spironolactone for acne in women: A case series of 403 patients Vaibhav Garg, BS,a JulianaK.Choi,MD,PhD,b William D. James, MD,b and John S. Barbieri, MD, MBAb Philadelphia, Pennsylvania Background: There are limited data regarding the long-term outcomes of spironolactone use for women with acne and its effect on truncal acne. Objective: To comprehensively describe outcomes of patients treated with spironolactone in routine clinical practice, including long-term outcomes. Methods: We performed a retrospective case series of 403 adult women treated for acne with spironolactone at an academic medical center between 2008 and 2019. Rates of objective, as assessed by Comprehensive Acne Severity Scale scores, and subjective acne clearance were evaluated, as well as rates of treatment discontinuation, dosage changes, and drug survival. Logistic regression was used to assess for association between incidence of menstrual adverse effects and combined oral contraceptive use. Results: As evaluated by Comprehensive Acne Severity Scale scores, at the first follow-up, 75.5%, 84.0%, and 80.2% of patients with available data had reduction or complete clearance of acne on the face, chest, and back, respectively. The mean drug survival was 470.7 days. Menstrual adverse effects were less common among those using combined oral contraception (odds ratio, 0.23; 95% confidence interval, 0.11-0.50). Limitations: This study was conducted at a single academic medical center. Conclusions: Spironolactone improves clinical outcomes and is well tolerated for many adult women with acne using it for an extended duration. ( J Am Acad Dermatol 2021;84:1348-55.) Key words: acne; acne vulgaris; birth control pill; combined oral contraceptive; comprehensive acne severity scale; oral antibiotics; outcomes; spironolactone. -

Neomycin—Production and Antibiotic Properties
NEOMYCIN—PRODUCTION AND ANTIBIOTIC PROPERTIES Selman A. Waksman, … , Hubert A. Lechevalier, Dale A. Harris J Clin Invest. 1949;28(5):934-939. https://doi.org/10.1172/JCI102182. Research Article Find the latest version: https://jci.me/102182/pdf NEOMYCIN-PRODUCTION AND ANTIBIOTIC PROPERTIES 1, 2 3 By SELMAN A. WAKSMAN, HUBERT A. LECHEVALIER, AND DALE A. HARRIS (From the Department of Microbiology, New Jersey Agricultural Experiment Station, Rutgers University, New Brunswick, N. J.) ANTIBIOTIC SURVEYS All these cultures proved to be highly active against mycobacteria. of During the last 10 years, a large number anti- Only few of the cultures, however, that gave biotics which are active against Gram-negative good activity by the agar-streak method yielded and Gram-positive bacteria, mycobacteria, rickett- filtrates which possessed corresponding potency. siae, and certain of the larger viruses were iso- This may be due to a variety of factors, such as lated (1) from various species and strains of the the formation by a single organism of more than genus Streptomyces. This served to focus atten- one antibiotic or the production of different anti- tion on the actinomycetes as potential producers of biotics under different conditions of culture. One antimicrobial agents that might possess promising of these cultures proved to be highly promising chemotherapeutic properties. The fact that nearly and was selected for more detailed investigations. 20 to 50%o of these organisms possess antimicrobial This culture was entered into the Collection as No. activities served to heighten this interest. Numer- 3535. The nature of its antimicrobial spectrum, ous surveys have been conducted. -
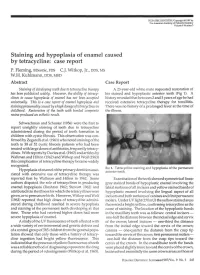
Staining and Hypoplasia of Enamel Caused by Tetracycline: Case Report P
PEDIATRIC DENTISTRY/Copyright © 1987 by The American Academy of Pediatric Dentistry Volume 9 Number 3 Staining and hypoplasia of enamel caused by tetracycline: case report P. Fleming, BDentSc, FDS C.J. Witkop, Jr., DDS, MS W.H. Kuhlmann, DDS, MSD Abstract Case Report Staining of developing teeth due to tetracycline therapy A 23-year-old white male requested restoration of has been publicized widely. However, the ability of tetracy- his stained and hypoplastic anterior teeth (Fig 1). A clines to cause hypoplasia of enamel has not been accepted history revealed that between 2 and 3 years of age he had universally. This is a case report of enamel hypoplasia and received extensive tetracycline therapy for tonsillitis. staining presumably caused byahigh dosage of'tetracy'dines in There was no history of a prolonged fever at the time of childhood. Restoration of the teeth with bonded composite the illness. resins produced an esthetic result. -UK Schwachman and Schuster (1956) were the first to report unsightly staining of teeth due to tetracycline administered during the period of tooth formation in children with cystic fibrosis. This observation was con- y firmed by Zegarelli et al. (1961) who noted staining of the teeth in 38 of 52 cystic fibrosis patients who had been ijgj treated with large doses of antibiotics, frequently tetracy- .^••W^^ clines. With reports by Davies et al. (1962) and articles by Wallman and Hilton (1962) and Witkop and Wolf (1963) this complication of tetracycline therapy became widely recognized. FIG 1. Tetracycline staining and hypoplasia of the permanent Hypoplasia of enamel of the primary dentition asso- anterior teeth. -

(ESVAC) Web-Based Sales and Animal Population
16 July 2019 EMA/210691/2015-Rev.2 Veterinary Medicines Division European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Sales Data and Animal Population Data Collection Protocol (version 3) Superseded by a new version Superseded Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Table of content 1. Introduction ....................................................................................................................... 3 1.1. Terms of reference ........................................................................................................... 3 1.2. Approach ........................................................................................................................ 3 1.3. Target groups of the protocol and templates ......................................................................... 4 1.4. Organization of the ESVAC project ...................................................................................... 4 1.5. Web based delivery of data ................................................................................................ 5 2. ESVAC sales data ............................................................................................................... 5 2.1. -

Topical Antibiotics
Topical antibiotics Microbiological point of view [email protected] • Topical antibiotics • Efficacy • Antibiotic resistance – Propionibacteria/acne • Tetracyclines • macrolides – Staphylococci/impetigo • Fusidic acid • Mupirocin • Epilogue Topical antibiotics in dermatology • Bacitracin • Chloramphénicol • Clindamycin • Erythromycin • Fusidic acid • Gentamicin • Miconazol • Mupirocin • Neomycin • Polymyxin B • Sulfamides • Tétracyclines • Benzoyle peroxyde • Azelaic acid + “bactéries (filtrat polyvalent) + huile de foie de morue 125 mg + sulfanilamide 200 mg/1 g “ Topical antibiotics in ophtalmology / ORL • Bacitracine • Chloramphénicol • Fusidic acid • Gentamicin • Gramicidin* • Quinolones: o-,nor-, cipro-, lome- floxacin* • Neomycin • Polymyxin B • Rifamycin* • Sulfamides • Tétracyclines • Trimethoprim* • Tobramycin* • Tyrothricine* Topical antibiotics: efficacy • Impetigo: mupirocin & fusidic acid*: + ** • Acne: + ~ benzoyl peroxyde • Chronic suppurative otitis media*: + ** • Acute bacterial conjunctivitis*: + * (Cochrane review) ** better than antiseptics Topical antibiotics: resistance • Propionibacterium spp: resistance to erythromycin: – mutation in the genes encoding 23S ribosomal RNA (3 phenotypes) resistance to tetracycline: – Mutation of the gene encoding 16S ribosomal RNA Propionibacteria Distribution and differentiation of human commensal propionibacterium P. acnes P. avidum P. granulosum P. propionicum distribution Skin +++ +++ +++ - Eye + - - +++ Mouth ++ - + +++ Gut +++ - - - differentiation Esculin - + - - Catalase +