Antioxidant Activity of Extracts Formulated from Citrus Aurantium and Artemisia Herba Alba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal Officiel N°2020-59
N° 59 Dimanche 16 Safar 1442 59ème ANNEE Correspondant au 4 octobre 2020 JJOOUURRNNAALL OOFFFFIICCIIEELL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANÇAISE) Algérie ETRANGER DIRECTION ET REDACTION Tunisie SECRETARIAT GENERAL ABONNEMENT Maroc (Pays autres DU GOUVERNEMENT ANNUEL Libye que le Maghreb) WWW.JORADP.DZ Mauritanie Abonnement et publicité: 1 An 1 An IMPRIMERIE OFFICIELLE Les Vergers, Bir-Mourad Raïs, BP 376 ALGER-GARE Edition originale................................... 1090,00 D.A 2675,00 D.A Tél : 021.54.35..06 à 09 Fax : 021.54.35.12 Edition originale et sa traduction.... 2180,00 D.A 5350,00 D.A C.C.P. 3200-50 Clé 68 ALGER (Frais d'expédition en sus) BADR : Rib 00 300 060000201930048 ETRANGER : (Compte devises) BADR : 003 00 060000014720242 Edition originale, le numéro : 14,00 dinars. Edition originale et sa traduction, le numéro : 28,00 dinars. Numéros des années antérieures : suivant barème. Les tables sont fournies gratuitement aux abonnés. Prière de joindre la dernière bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne 16 Safar 1442 2 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 59 4 octobre 2020 SOMMAIRE DECRETS Décret exécutif n° 20-274 du 11 Safar 1442 correspondant au 29 septembre 2020 modifiant et complétant le décret exécutif n° 96-459 du 7 Chaâbane 1417 correspondant au 18 décembre 1996 fixant les règles applicables aux -

Rapport Sur Les Priorités Et La Planification Année 2021
République Algérienne Démocratique et Populaire Ministère des Ressources en Eau Rapport sur les priorités et la planification Année 2021 Volume 2 Octobre/ 2020 Table des matières Contenu Section 1. Message du ministre .......................................................................................4 1.1 Message du ministre ...................................................................................................4 1.2 Déclaration du Secrétaire Général ..............................................................................5 Section 2. Au sujet du portefeuille...................................................................................6 2.1 La mission ...................................................................................................................6 - Production de l’eau domestique, industrielle et agricole, y compris la production et l’utilisation de l’eau de mer dessalée, de l’eau saumâtre et des eaux usées Épurées ;6 2.2 Le ministère ................................................................................................................7 2.3 Fiche Portefeuille ........................................................................................................9 Gestionnaire responsable : Ministre des Ressources en Eau ...............................................9 2.4 Planification des activités pour l’année 2021 ...........................................................11 Section 3. Planification détaillée du programme 01 ......................................................12 -

Journal Officiel N°2020-5
N° 05 Mercredi 4 Joumada Ethania 1441 59ème ANNEE Correspondant au 29 janvier 2020 JJOOUURRNNAALL OOFFFFIICCIIEELL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANÇAISE) Algérie ETRANGER DIRECTION ET REDACTION Tunisie SECRETARIAT GENERAL ABONNEMENT Maroc (Pays autres DU GOUVERNEMENT ANNUEL Libye que le Maghreb) WWW.JORADP.DZ Mauritanie Abonnement et publicité: IMPRIMERIE OFFICIELLE 1 An 1 An Les Vergers, Bir-Mourad Raïs, BP 376 ALGER-GARE Tél : 021.54.35..06 à 09 Edition originale................................... 1090,00 D.A 2675,00 D.A 021.65.64.63 Fax : 021.54.35.12 Edition originale et sa traduction.... 2180,00 D.A 5350,00 D.A C.C.P. 3200-50 ALGER TELEX : 65 180 IMPOF DZ (Frais d'expédition en sus) BADR : 060.300.0007 68/KG ETRANGER : (Compte devises) BADR : 060.320.0600 12 Edition originale, le numéro : 14,00 dinars. Edition originale et sa traduction, le numéro : 28,00 dinars. Numéros des années antérieures : suivant barème. Les tables sont fournies gratuitement aux abonnés. Prière de joindre la dernière bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne 2 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 05 4 Joumada Ethania 1441 29 janvier 2020 SOMMAIRE DECRETS Décret présidentiel n° 20-07 du 29 Joumada El Oula 1441 correspondant au 25 janvier 2020 fixant les attributions et l’organisation des services de la Présidence de la République............................................................................................................................. 4 Décret exécutif n° 19-391 du 4 Joumada El Oula 1441 correspondant au 31 décembre 2019 modifiant la répartition par secteur des dépenses d’équipement de l’Etat pour 2019........................................................................................................................................ -

Journal Officiel = De La Republique Algerienne Democratique Et Populaire Conventions Et Accords Internationaux - Lois Et Decrets
No 22 ~ Mercredi 14 Moharram 1421 ~ . 39 ANNEE correspondant au 19 avril 2000 Pee nls 43 Ub! sess Sbykelig bte é yr celyly S\,\n JOURNAL OFFICIEL = DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS. ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANCAISE) Algérie ; ER DIRECTION ET REDACTION: Tunisie ETRANGER SECRETARIAT GENERAL ABONNEMENT Maroc (Pays autres DU GOUVERNEMENT ANNUEL Libyeye que le Maghreb) ” , Mauritanie Abonnement et publicité: : IMPRIMERIE OFFICIELLE 1 An- 1 An 7,9 et 13 Av. A. Benbarek-ALGER Tél: 65.18.15 a 17 - C.C.P. 3200-50 | Edition originale.....ccccsesseeees 856,00 D.A| 2140,00 D.A _ ALGER Télex: 65 180 IMPOF DZ . BADR: 060.300.0007 68/KG Edition originale et sa traduction}1712,00 D.A|. .4280,00 D.A ETRANGER: (Compte devises): (Frais d'expédition en sus) BADR: 060.320.0600 12 Edition originale, le numéro : 10,00 dinars. Edition originale et sa traduction, le numéro : 20,00 dinars. Numéros des années antérieures : suivant baréme. Les tables sont fournies gratuitement aux abonnés. Priére de joindre la derniére bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne. JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 22.14 Moharram 1421 19 avril 2000 SOMMAIRE | | ; ARRETES, DECISIONS ET AVIS | MINISTERE DES FINANCES Arrété du 13 Ramadhan 1420 correspondant au 21 décembre 1999 modifiant et complétant l'arrété du 26 Rajab 1416 -correspondant au 19 décembre 1995 portant création des inspections des impéts dans les wilayas relevant de la _,direction régionale des imp6ts de Chlef... -

Helmut Honermann Aus Legden
Erkundung strategischer Konzepte des Erosionsschutzes und der Sedimentrückhaltung bei großen Speichern in semi-ariden Gebieten Algeriens Zur Erlangung des akademischen Grades eines DOKTOR-INGENIEURS von der Fakultät für Bauingenieur- und Vermessungswesen der Universität Fridericiana zu Karlsruhe (TH) genehmigte DISSERTATION von Dipl.-Ing. Helmut Honermann aus Legden Tag der mündlichen Prüfung: 23. Mai 2001 Hauptreferent: Univ.-Prof. Dr.-Ing. Werner Köhl Korreferenten: Univ.-Prof. Dr.sc.agr. Dieter Prinz Univ.-Prof. Dr.sc.techn. Bernd Scholl Karlsruhe: 2001 2 3 Kurzfassung Im Rahmen dieser Arbeit wurde für Zwecke der Regionalplanung eine Methodik zur Erkun- dung von Strategien des Erosionsschutzes und der Sedimentrückhaltung bei der Bewirtschaf- tung von Wassereinzugsgebieten großer Speicher erarbeitet. Sie gründet auf wirtschaftswis- senschaftlichen Kenntnissen und besitzt ihre fachliche Gültigkeit in Gebieten mit Mergeln und semi-aridem Klima bei einer gemischten Nutzungsform aus Getreideanbau und Viehwirt- schaft. Als praktisches Beispiel dient das Wassereinzugsgebiet des Staudamms Es Saada in Algerien. Ein Hauptgrund für den Bau großer Speicher in Algerien wie auch in anderen Ländern des Maghreb ist die Tatsache, daß das Wasserdargebot für die soziale und wirtschaftliche Ent- wicklung ein stark limitierender Faktor ist. Ursache für den gestiegenen Wasserbedarf ist das hohe Bevölkerungswachstum in den letzten 30 Jahren von im Mittel ca. 3,1 % pro Jahr. Durch eine Kette von Speichern dient praktisch der gesamte Tell Atlas als Wassereinzugsgebiet. Das Konzept der konzentrierten Wassernutzung wird somit für den nördlichen Teil Algeriens zu einer mehr oder weniger flächendeckenden Maßnahme. Viele dieser Speicher sind durch eine zunehmende Verlandung gekennzeichnet. Der hohe Sedimenttransport ist Folge von Linien- erosion in Mergeln. Der Verlust an Speicherkapazität reduziert die Rentabilität der Stau- dammvorhaben und gefährdet somit langfristig die politische Zielsetzung sowie das ökologi- sche, ökonomische und soziale Gleichgewicht. -

Aba Nombre Circonscriptions Électoralcs Et Composition En Communes De Siéges & Pourvoir
25ame ANNEE. — N° 44 Mercredi 29 octobre 1986 Ay\j SI AS gal ABAN bic SeMo, ObVel , - TUNIGIE ABONNEMENT ANNUEL ‘ALGERIE MAROC ETRANGER DIRECTION ET REDACTION: MAURITANIE SECRETARIAT GENERAL Abonnements et publicité : Edition originale .. .. .. .. .. 100 D.A. 150 DA. Edition originale IMPRIMERIE OFFICIELLE et satraduction........ .. 200 D.A. 300 DA. 7 9 et 13 Av. A. Benbarek — ALGER (frais d'expédition | tg}, ; 65-18-15 a 17 — C.C.P. 3200-50 ALGER en sus) Edition originale, le numéro : 2,50 dinars ; Edition originale et sa traduction, le numéro : 5 dinars. — Numéros des années antérleures : suivant baréme. Les tables sont fourntes gratul »ment aux abonnés. Priére dé joindre les derniéres bandes . pour renouveliement et réclamation. Changement d'adresse : ajouter 3 dinars. Tarif des insertions : 20 dinars la ligne JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX LOIS, ORDONNANCES ET DECRETS ARRETES, DECISIONS, CIRCULAIRES, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANGAISE) SOMMAIRE DECRETS des ceuvres sociales au ministére de fa protection sociale, p. 1230. Décret n° 86-265 du 28 octobre 1986 déterminant les circonscriptions électorales et le nombre de Décret du 30 septembre 1986 mettant fin aux siéges & pourvoir pour l’élection a l’Assemblée fonctions du directeur des constructions au populaire nationale, p. 1217. , ministére de la formation professionnelle et du travail, p. 1230. DECISIONS INDIVIDUELLES Décret du 30 septembre 1986 mettant fin aux fonctions du directeur général da la planification Décret du 30 septembre 1986 mettant fin aux et de. la gestion industrielle au ministére de fonctions du directeur de la sécurité sociale et lindustrie lourde,.p. -

Plan Développement Réseau Transport Gaz Du GR TG 2017 -2027 En Date
Plan de développement du Réseau de Transportdu Gaz 2014-2024 N°901- PDG/2017 N°480- DOSG/2017 CA N°03/2017 - N°021/CA/2017 Mai 2017 Plan de développement du Réseau de Transport du Gaz 2017-2027 Sommaire INTRODUCTION I. SYNTHESE DU PLAN DE DEVELOPPEMENT I.1. Synthèse physique des ouvrages I.2. Synthèse de la valorisation de l’ensemble des ouvrages II. PROGRAMME DE DEVELOPPEMENT DES RESEAUX GAZ II.1. Ouvrages mis en gaz en 201 6 II.2. Ouvrages alimentant la Wilaya de Tamanrasset et Djanet II.3. Ouvrages Infrastructurels liés à l’approvisionnement en gaz nature l II.4. Ouvrages liés au Gazoduc Rocade Est -Ouest (GREO) II.5. Ouvrages liés à la Production d’Electricité II.6. Ouvrages liés aux Raccordement de la C lientèle Industrielle Nouvelle II.7. Ouvrages liés aux Distributions Publiques du gaz II .8. Ouvrages gaz à réhabiliter II. 9. Ouvrages à inspecter II. 10 . Plan Infrastructure II.1 1. Dotation par équipement du Centre National de surveillance II.12 . Prévisions d’acquisition d'équipements pour les besoins d'exploitation II.13 .Travaux de déviation des gazoducs Haute Pression II.1 4. Ouvrages en idée de projet non décidés III. BILAN 2005 – 201 6 ET PERSPECTIVES 201 7 -202 7 III.1. Evolution du transit sur la période 2005 -2026 III.2. Historique et perspectives de développement du ré seau sur la période 2005 – 202 7 ANNEXES Annexe 1 : Ouvrages mis en gaz en 201 6 Annexe 2 : Distributions Publiques gaz en cours de réalisation Annexe 3 : Distributions Publiques gaz non entamées Annexe 4 : Renforcements de la capacité des postes DP gaz Annexe 5 : Point de situation sur le RAR au 30/04/2017 Annexe 6 : Fibre optique sur gazoducs REFERENCE Page 2 Plan de développement du Réseau de Transport du Gaz 2017-2027 INTRODUCTION : Ce document a pour objet de donner le programme de développement du réseau du transport de gaz naturel par canalisations de la Société Algérienne de Gestion du Réseau de Transport du Gaz (GRTG) sur la période 2017-2027. -

Journal Officiel Algérie
N° 01 Mercredi 20 Moharram 1431 49ème ANNEE Correspondant au 6 janvier 2010 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANÇAISE) DIRECTION ET REDACTION Algérie ETRANGER SECRETARIAT GENERAL Tunisie (Pays autres DU GOUVERNEMENT ABONNEMENT Maroc que le Maghreb) ANNUEL Libye WWW. JORADP. DZ Mauritanie Abonnement et publicité: IMPRIMERIE OFFICIELLE 1 An 1 An Les Vergers, Bir-Mourad Raïs, BP 376 ALGER-GARE Tél : 021.54.35..06 à 09 Edition originale….........….........…… 1070,00 D.A 2675,00 D.A 021.65.64.63 Fax : 021.54.35.12 Edition originale et sa traduction....... 2140,00 D.A 5350,00 D.A C.C.P. 3200-50 ALGER (Frais d'expédition en TELEX : 65 180 IMPOF DZ BADR: 060.300.0007 68/KG sus) ETRANGER: (Compte devises) BADR: 060.320.0600 12 Edition originale, le numéro : 13,50 dinars. Edition originale et sa traduction, le numéro : 27,00 dinars. Numéros des années antérieures : suivant barème. Les tables sont fournies gratuitement aux abonnés. Prière de joindre la dernière bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne 2 20 Moharram 1431 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 01 6 janvier 2010 S O M M A I R E DECRETS Décret exécutif n° 10-01 du 18 Moharram 1431 correspondant au 4 janvier 2010 relatif au plan directeur d’aménagement des ressources en eau et au plan national de l’eau........................................................................................................................... 3 Décret exécutif n° 10-02 du 18 Moharram 1431 correspondant au 4 janvier 2010 fixant les dispositions relatives à l'obligation de l'enseignement fondamental................................................................................................................................................ -

Poste De Transformation Adrar 400/220 Kv - (Adrar)
19 Ramadhan 1434 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 38 28 juillet 2013 7 Décret exécutif n° 13-268 du 12 Ramadhan 1434 1- Poste de transformation Adrar 400/220 kV - (Adrar). correspondant au 21 juillet 2013 portant déclaration d'utilité publique l'opération relative 2- Poste de transformation Reggane 220/60 kV - à la réalisation des postes de transformation (Adrar). haute et très haute tension. 3- Poste de transformation Adrar 1 60/30 kV - (Adrar). ———— 4- Poste de transformation Adrar II 220/60 kV - Le Premier ministre, (Adrar). Sur le rapport du ministre de l'énergie et des mines, 5- Poste de transformation Adrar II 60/30 kV - (Adrar). Vu la Constitution, notamment ses articles 85-3° et 125 6- Poste de transformation Timimoun 220/60 kV - (alinéa 2) ; (Adrar). Vu la loi n° 90-30 du 1er décembre 1990, modifiée et 7- Poste de transformation Adrar III 60/30 kV - (Adrar). complétée, portant loi domaniale ; 8- Poste de transformation Kaberten 220/60 kV - Vu la loi n° 91-11 du 27 avril 1991, complétée, fixant (Adrar). les règles relatives à l'expropriation pour cause d'utilité publique ; 9- Poste de transformation Adrar IV 60/30 kV - (Adrar). Vu la loi n° 98-04 du 20 Safar 1419 correspondant 10- Poste de transformation Zaouiet Kounta 220/60 kV - au 15 juin 1998 relative à la protection du patrimoine (Adrar). culturel ; 11- Poste de transformation Adrar V 60/30 kV - Vu la loi n° 02-01 du 22 Dhou El Kaâda 1422 (Adrar). correspondant au 5 février 2002 relative à l'électricité et à la distribution du gaz par canalisations ; 12- Poste de transformation Oued Sly II 220/400 kV - (Chlef). -

Mémoire Thème Traitement Et Intégration Des Données
REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE MINISTERE DE L’ENSEIGNEMENT SUPERIEUR ET DE LA RECHERCHE SCIENTIFIQUE UNIVERSITE ABDEL HAMID BEN BADIS MOSTAGANEM Mémoire En vue de l’obtention du diplôme de Master Département: Science d’agronomie Thème Traitement et Intégration des données météorologiques dans un SIG pour la détection des changements climatiques du sous bassin versant d’’ Oued Mina Haddad Présenté par : BOUMEDIENE Mebarek M. Rguieg Y Laarbi : Président M. Ferah Tahar : Examinateur M. Hartani Ahmed : Encadreur REMERCIEMENT A la fin de ce modeste travail, c’est pour nous un grand honneur et un réel plaisir de rendre hommage, témoigner notre profonde reconnaissance et formuler des remerciements aux personnes qui, d’une manière ou d’une autre, ont apporté leur soutien et contribué à sa réalisation. En premier lieu, nous adressons nos sincère remerciement à Monsieur Hartani Ahmed. Maitre de conférence à université de Mostaganem qui nous a proposé ce sujet et nous a guidé et encouragé tout au long de l’avancement de ce travail. Nous lui témoignons notre très vive et respectueuse gratitude. Nous remercions également très chaleureusement Monsieur Baghdadi, Attaché de Recherche, qui nous a fait l’honneur de nous encadrer tout au long de ce travail de mémoire. Nous remercions aussi très vivement Monsieur Rguieg Laarbi , le professeur de l'université de Mostaganem, de nous avoir fait l’honneur de présider le jury ainsi que Monsieur Farah Tahar, Attaché de Recherche au Centre National des Technique Spatial d’Arzew et, qui ont bien voulu examiner ce travail. Enfin nous sincère remerciements à nos camarades étudiants pour leur soutien moral et l’aide qu’ils nous ont toujours apporté. -
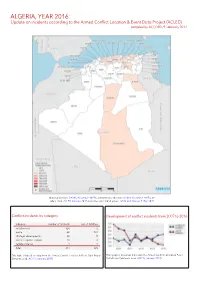
Algeria, Year 2016: Update on Incidents According to the Armed Conflict Location & Event Data Project (ACLED)
ALGERIA, YEAR 2016: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) compiled by ACCORD, 9 February 2017 National borders: GADM, November 2015b; administrative divisions: GADM, November 2015a; in- cident data: ACLED, January 2017; coastlines and inland waters: Smith and Wessel, 1 May 2015 Conflict incidents by category Development of conflict incidents from 2007 to 2016 category number of incidents sum of fatalities riots/protests 324 2 battle 48 122 strategic developments 28 0 violence against civilians 10 4 remote violence 4 1 total 414 129 This table is based on data from the Armed Conflict Location & Event Data Project This graph is based on data from the Armed Conflict Location & Event (datasets used: ACLED, January 2017). Data Project (datasets used: ACLED, January 2017). ALGERIA, YEAR 2016: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 9 FEBRUARY 2017 LOCALIZATION OF CONFLICT INCIDENTS Note: The following list is an overview of the incident data included in the ACLED dataset. More details are available in the actual dataset (date, location data, event type, involved actors, information sources, etc.). In the following list, the names of event locations are taken from ACLED, while the administrative region names are taken from GADM data which serves as the basis for the map above. In Adrar, 11 incidents killing 0 people were reported. The following locations were affected: Adrar, Aougrout, Bordj Badji Mokhtar. In Alger, 34 incidents killing 0 people were reported. The following locations were affected: Ain Benian, Algiers, Bab El Oued, Draria, El Hamiz, Rouiba. -

Entre Retard De Livraison Et Chantiers À L'arrêt
L’Algérie profonde / Ouest Réalisation des programmes de logements à Mascara Entre retard de livraison et chantiers à l’arrêt Les souscr ipteur s toujou rs dans l’atten te des clefs de leurs logem ents. © D.R. Des centaines de chantiers sont en souffrance, au moment où des milliers de chefs de famille revendiquent un toit. En effet, la wilaya enregistre depuis ces trois dernières décennies et à intervalles réguliers différents programmes toutes formules confondues relatifs à la réalisation de logements à même de résoudre ou du moins atténuer la crise qui se pose avec acuité dans la région. Mais le non-respect des délais de livraison, aggravé par une démographie galopante, n’a pas permis aux autorités ayant pouvoir de décision d’accéder aux revendications des citoyens sans abri, de ceux vivant à l’étroit ou de ceux qui réclament un toit pour fonder un foyer. Dans ce contexte, il y a lieu de relever que des milliers de demandeurs de logement attendent avec impatience depuis 2011 la régularisation de leurs cas sans obtenir satisfaction, ce qui débouche sur une manifestation chaque jour de réception en se rendant massivement au niveau des sièges des différentes daïras de la wilaya. Certains chefs de famille fragilisés mentalement font usage de menaces et de tentatives de suicide, des actes de désespoir considérés comme dernier recours. Ainsi, pour combler le déficit dans le secteur, des chantiers de construction de bâtiments sont installés un peu partout dans les communes de la wilaya de Mascara, des efforts axés principalement sur les grandes agglomérations. Ainsi, un programme de 80 logements LPA confié à une entreprise privée est en cours de réalisation dans la commune de Mascara.