You Cannot Have It All: Heritability and Constraints of Predator‐
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
For Review Only
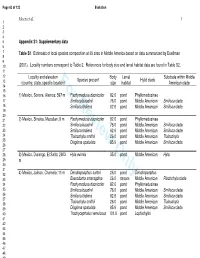
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Mannophryne Olmonae) Catherine G
The College of Wooster Libraries Open Works Senior Independent Study Theses 2014 A Not-So-Silent Spring: The mpI acts of Traffic Noise on Call Features of The loB ody Bay Poison Frog (Mannophryne olmonae) Catherine G. Clemmens The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Environmental Sciences Commons Recommended Citation Clemmens, Catherine G., "A Not-So-Silent Spring: The mpI acts of Traffico N ise on Call Features of The loodyB Bay Poison Frog (Mannophryne olmonae)" (2014). Senior Independent Study Theses. Paper 5783. https://openworks.wooster.edu/independentstudy/5783 This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The oC llege of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2014 Catherine G. Clemmens A NOT-SO-SILENT SPRING: THE IMPACTS OF TRAFFIC NOISE ON CALL FEATURES OF THE BLOODY BAY POISON FROG (MANNOPHRYNE OLMONAE) DEPARTMENT OF BIOLOGY INDEPENDENT STUDY THESIS Catherine Grace Clemmens Adviser: Richard Lehtinen Submitted in Partial Fulfillment of the Requirement for Independent Study Thesis in Biology at the COLLEGE OF WOOSTER 2014 TABLE OF CONTENTS I. ABSTRACT II. INTRODUCTION…………………………………………...............…...........1 a. Behavioral Effects of Anthropogenic Noise……………………….........2 b. Effects of Anthropogenic Noise on Frog Vocalization………………....6 c. Why Should We Care? The Importance of Calling for Frogs..................8 d. Color as a Mode of Communication……………………………….…..11 e. Biology of the Bloody Bay Poison Frog (Mannophryne olmonae)…...13 III. -

Dedicated to the Conservation and Biological Research of Costa Rican Amphibians”
“Dedicated to the Conservation and Biological Research of Costa Rican Amphibians” A male Crowned Tree Frog (Anotheca spinosa) peering out from a tree hole. 2 Text by: Brian Kubicki Photography by: Brian Kubicki Version: 3.1 (October 12th, 2009) Mailing Address: Apdo. 81-7200, Siquirres, Provincia de Limón, Costa Rica Telephone: (506)-8889-0655, (506)-8841-5327 Web: www.cramphibian.com Email: [email protected] Cover Photo: Mountain Glass Frog (Sachatamia ilex), Quebrada Monge, C.R.A.R.C. Reserve. 3 Costa Rica is internationally recognized as one of the most biologically diverse countries on the planet in total species numbers for many taxonomic groups of flora and fauna, one of those being amphibians. Costa Rica has 190 species of amphibians known from within its tiny 51,032 square kilometers territory. With 3.72 amphibian species per 1,000 sq. km. of national territory, Costa Rica is one of the richest countries in the world regarding amphibian diversity density. Amphibians are under constant threat by contamination, deforestation, climatic change, and disease. The majority of Costa Rica’s amphibians are surrounded by mystery in regards to their basic biology and roles in the ecology. Through intense research in the natural environment and in captivity many important aspects of their biology and conservation can become better known. The Costa Rican Amphibian Research Center (C.R.A.R.C.) was established in 2002, and is a privately owned and operated conservational and biological research center dedicated to studying, understanding, and conserving one of the most ecologically important animal groups of Neotropical humid forest ecosystems, that of the amphibians. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species. -

Responses to Nonlethal Predators and the Roles of Competition and Resource Use
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 SPECIES LEVEL DIFFERENCES IN THE ECOLOGY OF TWO NEOTROPICAL TADPOLE SPECIES: RESPONSES TO NONLETHAL PREDATORS AND THE ROLES OF COMPETITION AND RESOURCE USE Zacharia Costa Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Biology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2635 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. © Zacharia J. Costa All Rights Reserved SPECIES LEVEL DIFFERENCES IN THE ECOLOGY OF TWO NEOTROPICAL TADPOLE SPECIES: RESPONSES TO NONLETHAL PREDATORS AND THE ROLES OF COMPETITION AND RESOURCE USE A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE AT VIRGINIA COMMONWEALTH UNIVERSITY by ZACHARIA J. COSTA Bachelor of Science, University of California, Davis 2007 Director DR. JAMES VONESH DEPARTMENT OF BIOLOGY Virginia Commonwealth University Richmond, Virginia December 13, 2011 Acknowledgments This would not have been possible if not for the support of my parents Kim and Marty Costa. They have always encouraged my pursuit of knowledge and love of the natural world, and are thus directly responsible for my career as a biologist. They have supported me in all of my endeavors often at their own expense, financially and emotionally, and for that I will forever be grateful. I also want to express my deep gratitude and thanks to my advisor Dr. -

Common FROGS of La Selva Biological Station, Costa Rica
Common FROGS of La Selva Biological Station, Costa Rica 1 2 3 3 1 1 Michelle E. Thompson , Juan G. Abarca , Enrique Salicetti Nelson , Hansell Rodriguez Vega, Carlos de la Rosa , Ralph Garcia Robleto, Maureen A. Donnelly 1Florida International University, 2Universidad de Costa Rica, 3Organization for Tropical Studies Photos: Juan G. Abarca (JGA), Carlos de la Rosa (CdlR), Ralph Garcia Robleto (RGR), Hansell Rodriguez Vega (HRV), Enrique Salicetti Nelson (ESN), Michelle E. Thompson (MET) Produced by: Michelle E. Thompson. Thanks to: José Iván Castillo Gomez and Selva Verde Lodge © Michelle E. Thompson [[email protected]] (M) Male, (Juv.) Juvenile; Lengths (Savage 2002) [fieldguides.fieldmuseum.org] [860] version 1 02/2017 1 Incilius melanochlorus 2 Rhaebo haematiticus 3 Rhinella marina 4 Espadarana prosoblepon (M) <104mm photo: ESN <81mm photo: CdlR <176mm photo: MET <32mm photo: MET BUFONIDAE BUFONIDAE BUFONIDAE CENTROLENIDAE 5 Hyalinobatrachium fleischmanni 6 Hyalinobatrachium valerioi 7 Teratohyla spinosa 8 Craugastor bransfordii <32mm photo: MET <27mm photo: ESN <24mm photo: JGA <27mm photo: ESN CENTROLENIDAE CENTROLENIDAE CENTROLENIDAE CRAUGASTORIDAE 9 Craugastor crassidigitus 10 Craugastor fitzingeri 11 Craugastor fitzingeri 12 Craugastor megacephalus <27mm photo: HRV <54mm photo: MET <54mm photo: MET <71mm photo: MET CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE 13 Craugastor mimus 14 Craugastor noblei 15 Craugastor talamancae 16 Diasporus diastema <59mm photo: JGA <67mm photo: MET <51mm photo: JGA <25mm photo: JGA CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE 17 Pristimantis cerasinus 18 Pristimantis cruentus 19 Pristimantis ridens 20 Dendrobates auratus <36mm photo: HRV <43mm photo: MET <26mm photo: ESN <43mm photo: MET CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE DENDROBATIDAE Common FROGS of La Selva Biological Station, Costa Rica 1 2 3 3 1 2 Michelle E. -

Variation in Abundance and Efficacy of Tadpole Predators in a Neotropical Pond Community Author(S): Justin C
Variation in Abundance and Efficacy of Tadpole Predators in a Neotropical Pond Community Author(s): Justin C. Touchon and James R. Vonesh Source: Journal of Herpetology, 50(1):113-119. Published By: The Society for the Study of Amphibians and Reptiles DOI: http://dx.doi.org/10.1670/14-111 URL: http://www.bioone.org/doi/full/10.1670/14-111 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Herpetology, Vol. 50, No. 1, 113–119, 2016 Copyright 2016 Society for the Study of Amphibians and Reptiles Variation in Abundance and Efficacy of Tadpole Predators in a Neotropical Pond Community 1,3 2 JUSTIN C. TOUCHON AND JAMES R. VONESH 1Boston University, Department of Biology, 5 Cummington St., Boston, Massachusetts USA 2Virginia Commonwealth University, Department of Biology, 1000 West Cary Street, Richmond, Virginia USA ABSTRACT.—Variation in predation risk plays an important role in shaping prey behavior, morphology, life history, population dynamics, and community structure in freshwater systems. -

Amphibians of the CRARC
Herein we are following the familiar, generic, and specific arrangements of Amphibiaweb, June 2012. Version 1.0 (June 2012) Mailing Address: Apdo. 81-7200, Siquirres, Provincia de Limón, Costa Rica Telephone: (506)-8889-0655, (506)-8841-5327 Web: www.cramphibian.com Email: [email protected] Find us on Facebook: Costa Rican Amphibian Research Center 70 60 50 40 66 30 54 48 48 40 20 38 33 32 32 30 30 10 18 0 Osa Penas Carara Rara Avis La Suerte Guayacan Tortugero C.R.A.R.C. Las Cruces Santa Rosa San Ramon La Selva B.S. Amphibian diversity at various sites in Costa Rica. 20 19 15 10 8 7 5 5 3 3 2 2 2 2 1 Number of Species 0 Hylidae Ranidae Bufonidae Dermophiidae Centrolenidae Dendrobatidae Plethodontidae Craugastoridae Leptodactylidae Strabomantidae Eleutherodactylidae Representation of Species Diversity per Family for the Class Ampibia at the C.R.A.R.C. Reserve. 2 Amphibian Species of the C.R.A.R.C. Reserve: Order: Gymnophiona Oophaga pumilio Family: Dermophiidae Phyllobates lugubris Dermophis parviceps Silverstoneia flotator Gymnopis multiplicata Family: Eleutherodactylidae Diasporus diastema Order: Caudata Family: Plethodontidae Family: Hylidae Bolitoglossa colonnea Agalychnis callidryas B. striatula A. lemur A. saltator Order: Anura A. spurrelli Anotheca spinosa Family: Bufonidae Cruziohyla calcarifer Incilius melanochlorus Dendropsophus ebraccatus Rhaebo haematiticus D. phlebodes Rhinella marina Duellmanohyla rufioculis D. uranochroa Family Centrolenidae Ecnomiohyla sukia Cochranella granulosa Hyloscirtus palmeri Hyalinobatrachium fleischmanni Isthmohyla lancasteri H. talamancae Scinax boulengeri H. valerioi S. elaeochrous Sachatamia albomaculata Smilisca baudinii S. ilex S. phaeota Teratohyla pulverata S. sordida T. spinosa Tlalocohyla loquax Family: Craugastoridae Family: Leptodactylidae Craugastor bransfordii Leptodactylus melanonotus C. -

Systematic Review of the Frog Family Hylidae, with Special Reference to Hylinae: Phylogenetic Analysis and Taxonomic Revision
SYSTEMATIC REVIEW OF THE FROG FAMILY HYLIDAE, WITH SPECIAL REFERENCE TO HYLINAE: PHYLOGENETIC ANALYSIS AND TAXONOMIC REVISION JULIAÂ N FAIVOVICH Division of Vertebrate Zoology (Herpetology), American Museum of Natural History Department of Ecology, Evolution, and Environmental Biology (E3B) Columbia University, New York, NY ([email protected]) CEÂ LIO F.B. HADDAD Departamento de Zoologia, Instituto de BiocieÃncias, Unversidade Estadual Paulista, C.P. 199 13506-900 Rio Claro, SaÄo Paulo, Brazil ([email protected]) PAULO C.A. GARCIA Universidade de Mogi das Cruzes, AÂ rea de CieÃncias da SauÂde Curso de Biologia, Rua CaÃndido Xavier de Almeida e Souza 200 08780-911 Mogi das Cruzes, SaÄo Paulo, Brazil and Museu de Zoologia, Universidade de SaÄo Paulo, SaÄo Paulo, Brazil ([email protected]) DARREL R. FROST Division of Vertebrate Zoology (Herpetology), American Museum of Natural History ([email protected]) JONATHAN A. CAMPBELL Department of Biology, The University of Texas at Arlington Arlington, Texas 76019 ([email protected]) WARD C. WHEELER Division of Invertebrate Zoology, American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 294, 240 pp., 16 ®gures, 2 tables, 5 appendices Issued June 24, 2005 Copyright q American Museum of Natural History 2005 ISSN 0003-0090 CONTENTS Abstract ....................................................................... 6 Resumo ....................................................................... -

Capa 01 Phyllomedusa 7-1 NOVA.Cdr
Phyllomedusa 7(1):25-33, 2008 © 2008 Departamento de Ciências Biológicas - ESALQ - USP ISSN 1519-1397 Helminths from fifteen species of frogs (Anura, Hylidae) from Costa Rica Stephen R. Goldberg1 and Charles R. Bursey2 1 Whittier College, Department of Biology, Whittier, California 90608, USA. E-mail: [email protected]. 2 Pennsylvania State University, Department of Biology, Shenango Campus, Sharon, Pennsylvania 16146, USA. Abstract Helminths from fifteen species of frogs (Anura, Hylidae) from Costa Rica. Fifteen species of Costa Rican hylid frogs were examined for helminths: Agalychnis annae, Agalychnis callidryas, Agalychnis spurrelli, Dendropsophus ebraccatus, Dendropso- phus phlebodes, Duellmanohyla uranochroa, Hylomantis lemur, Hypsiboas rosenbergi, Isthmohyla pictipes, Isthmohyla rivularis, Isthmohyla tica, Scinax elaeochrous, Smilisca phaeota, Smilisca sordida, Tlalocohyla loquax. The frogs were found to harbor twelve species of helminths including one species of Monogenea, (Polystoma naevius), two species of Digenea (Gorgoderina diaster and Mesocoelium monas), eight species of Nematoda (Cosmocerca podicipinus, Falcaustra costaricae, Ochoterenella digiticauda, Oswaldocruzia costaricensis, Oxyascaris mcdiarmidi, Rhabdias savagei, Physaloptera sp. and Acuariidae gen. sp.) and one species of Acanthocephala (Anuracanthorhynchus lutzi). Mean number of helminth species per infected host species was 2.7 ± 0.3 SE (range 1-5). Thirty-nine new host records are reported. Keywords: Anura, Hylidae, helminths, Monogenea, Digenea, Nematoda, Acantho- cephala, Costa Rica. Introduction Brooks (2004) described Parapharyngodon duniae from Phrynohyas venulosa (currently The helminth biodiversity of Neotropical Trachycephalus venulosus). Brooks et al. vertebrates is virtually unknown (Salgado- (2006) listed platyhelminth parasites from 6 Maldonado et al. 2000) and consequently little Costa Rican hylid species, Isthmohyla information is available on the helminths of lancasteri, Scinax boulengeri, Smilisca baudinii, Costa Rican hylid frogs. -

Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae)
Zootaxa 4104 (1): 001–109 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4104.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:D598E724-C9E4-4BBA-B25D-511300A47B1D ZOOTAXA 4104 Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae) WILLIAM E. DUELLMAN1,3, ANGELA B. MARION2 & S. BLAIR HEDGES2 1Biodiversity Institute, University of Kansas, 1345 Jayhawk Blvd., Lawrence, Kansas 66045-7593, USA 2Center for Biodiversity, Temple University, 1925 N 12th Street, Philadelphia, Pennsylvania 19122-1601, USA 3Corresponding author. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by M. Vences: 27 Oct. 2015; published: 19 Apr. 2016 WILLIAM E. DUELLMAN, ANGELA B. MARION & S. BLAIR HEDGES Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae) (Zootaxa 4104) 109 pp.; 30 cm. 19 April 2016 ISBN 978-1-77557-937-3 (paperback) ISBN 978-1-77557-938-0 (Online edition) FIRST PUBLISHED IN 2016 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/j/zt © 2016 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. -

Differences in the Diversity of Frogspecies Between Sierra Lloronaand El Valle, Panama Kei Okabe Thurber SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 12-6-2014 Differences in the Diversity of FrogSpecies between Sierra Lloronaand El Valle, Panama Kei Okabe Thurber SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Biodiversity Commons, Environmental Health and Protection Commons, Environmental Indicators and Impact Assessment Commons, Latin American Studies Commons, Other Animal Sciences Commons, Population Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Thurber, Kei Okabe, "Differences in the Diversity of FrogSpecies between Sierra Lloronaand El Valle, Panama" (2014). Independent Study Project (ISP) Collection. 2000. https://digitalcollections.sit.edu/isp_collection/2000 This Article is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Differences in the Diversity of Frog Species between Sierra Llorona and El Valle, Panama By Kei Thurber SIT Panama, Fall 2014 Thurber 2 Table of Contents Acknowledgements ................................................................. 3 Abstract ................................................................................... 4 Resumen ................................................................................