Responses to Nonlethal Predators and the Roles of Competition and Resource Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tadpole Consumption Is a Direct Threat to the Endangered Purple Frog, Nasikabatrachus Sahyadrensis
SALAMANDRA 51(3) 252–258 30 October 2015 CorrespondenceISSN 0036–3375 Correspondence Tadpole consumption is a direct threat to the endangered purple frog, Nasikabatrachus sahyadrensis Ashish Thomas & S. D. Biju Systematics Lab, Department of Environmental Studies, University of Delhi, Delhi 110 007, India Corresponding author: S. D. Biju, e-mail: [email protected] Manuscript received: 5 July 2014 Accepted: 30 December 2014 by Alexander Kupfer Amphibians across the world are suffering alarming popu- Southeast Asia have witnessed drastic population declines lation declines with nearly one third of the ca 7,300 species caused by overexploitation over the last couple of decades being threatened worldwide (Stuart et al. 2008, Wake & (Warkentin et al. 2008). Often, natural populations are Vredenburg 2008, IUCN 2014). Major factors attributed harvested without regard of the consequences or implica- to the decline include habitat destruction (Houlahan et tions of this practice on the dynamics or sustainability of al. 2000, Sodhi et al. 2008), chemical pollution (Ber ger the exploited populations (Getz & Haight 1989). When 1998), climate change (Adams 1999, Carpenter & Tur- the extent of exploitation is greater than the sustaining ca- ner 2000), diseases (McCallum 2007, Cushman 2006), pacity or turnover rate of a species, there is every possi- and invasive species (Boone & Bridges 2003). The West- bility that the species may become locally extinct, which ern Ghats of India, a global hotspot for amphibian diver- would subsequently have drastic ecological implications sity and endemism (Biju 2001, Biju & Bossuyt 2003), has in the particular region (Duffy 2002, Wright et al. 2006, more than 40% of its amphibian fauna threatened with ex- Carpenter et al. -

What Do Tadpoles Really Eat? Assessing the Trophic Status of an Understudied and Imperiled Group of Consumers in Freshwater Habitats
Freshwater Biology (2007) 52, 386–395 doi:10.1111/j.1365-2427.2006.01694.x OPINION What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats RONALD ALTIG,* MATT R. WHILES† AND CINDY L. TAYLOR‡ *Department of Biological Sciences, Mississippi State University, Mississippi State, MS, U.S.A. †Department of Zoology and Center for Ecology, Southern Illinois University, Carbondale, IL, U.S.A. ‡Department of Biology, Austin Peay State University, Clarksville, TN, U.S.A. SUMMARY 1. Understanding the trophic status of consumers in freshwater habitats is central to understanding their ecological roles and significance. Tadpoles are a diverse and abundant component of many freshwater habitats, yet we know relatively little about their feeding ecology and true trophic status compared with many other consumer groups. While many tadpole species are labelled herbivores or detritivores, there is surprisingly little evidence to support these trophic assignments. 2. Here we discuss shortcomings in our knowledge of the feeding ecology and trophic status of tadpoles and provide suggestions and examples of how we can more accurately quantify their trophic status and ecological significance. 3. Given the catastrophic amphibian declines that are ongoing in many regions of the planet, there is a sense of urgency regarding this information. Understanding the varied ecological roles of tadpoles will allow for more effective conservation of remaining populations, benefit captive breeding programmes, and allow for more accurate predic- tions of the ecological consequences of their losses. Keywords: amphibian, assimilation, diet, feeding behaviour, omnivory Amphibians are disappearing from the planet at an of the functional roles and trophic status of general- alarming rate (Stuart et al., 2004; Lips et al., 2005). -

A New Species of the Genus Nasikabatrachus (Anura, Nasikabatrachidae) from the Eastern Slopes of the Western Ghats, India
Alytes, 2017, 34 (1¢4): 1¢19. A new species of the genus Nasikabatrachus (Anura, Nasikabatrachidae) from the eastern slopes of the Western Ghats, India S. Jegath Janani1,2, Karthikeyan Vasudevan1, Elizabeth Prendini3, Sushil Kumar Dutta4, Ramesh K. Aggarwal1* 1Centre for Cellular and Molecular Biology (CSIR-CCMB), Uppal Road, Tarnaka, Hyderabad, 500007, India. <[email protected]>, <[email protected]>. 2Current Address: 222A, 5th street, Annamalayar Colony, Sivakasi, 626123, India.<[email protected]>. 3Division of Vertebrate Zoology, Department of Herpetology, American Museum of Natural History, Central Park West at 79th Street, New York NY 10024-5192, USA. <[email protected]>. 4Nature Environment and Wildlife Society (NEWS), Nature House, Gaudasahi, Angul, Odisha. <[email protected]>. * Corresponding Author. We describe a new species of the endemic frog genus Nasikabatrachus,from the eastern slopes of the Western Ghats, in India. The new species is morphologically, acoustically and genetically distinct from N. sahyadrensis. Computed tomography scans of both species revealed diagnostic osteological differences, particularly in the vertebral column. Male advertisement call analysis also showed the two species to be distinct. A phenological difference in breeding season exists between the new species (which breeds during the northeast monsoon season; October to December), and its sister species (which breeds during the southwest monsoon; May to August). The new species shows 6 % genetic divergence (K2P) at mitochondrial 16S rRNA (1330 bp) partial gene from its congener, indicating clear differentiation within Nasikabatra- chus. Speciation within this fossorial lineage is hypothesized to have been caused by phenological shift in breeding during different monsoon seasons—the northeast monsoon in the new species versus southwest monsoon in N. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -
For Review Only
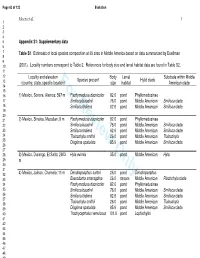
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Mannophryne Olmonae) Catherine G
The College of Wooster Libraries Open Works Senior Independent Study Theses 2014 A Not-So-Silent Spring: The mpI acts of Traffic Noise on Call Features of The loB ody Bay Poison Frog (Mannophryne olmonae) Catherine G. Clemmens The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Environmental Sciences Commons Recommended Citation Clemmens, Catherine G., "A Not-So-Silent Spring: The mpI acts of Traffico N ise on Call Features of The loodyB Bay Poison Frog (Mannophryne olmonae)" (2014). Senior Independent Study Theses. Paper 5783. https://openworks.wooster.edu/independentstudy/5783 This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The oC llege of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2014 Catherine G. Clemmens A NOT-SO-SILENT SPRING: THE IMPACTS OF TRAFFIC NOISE ON CALL FEATURES OF THE BLOODY BAY POISON FROG (MANNOPHRYNE OLMONAE) DEPARTMENT OF BIOLOGY INDEPENDENT STUDY THESIS Catherine Grace Clemmens Adviser: Richard Lehtinen Submitted in Partial Fulfillment of the Requirement for Independent Study Thesis in Biology at the COLLEGE OF WOOSTER 2014 TABLE OF CONTENTS I. ABSTRACT II. INTRODUCTION…………………………………………...............…...........1 a. Behavioral Effects of Anthropogenic Noise……………………….........2 b. Effects of Anthropogenic Noise on Frog Vocalization………………....6 c. Why Should We Care? The Importance of Calling for Frogs..................8 d. Color as a Mode of Communication……………………………….…..11 e. Biology of the Bloody Bay Poison Frog (Mannophryne olmonae)…...13 III. -

Dedicated to the Conservation and Biological Research of Costa Rican Amphibians”
“Dedicated to the Conservation and Biological Research of Costa Rican Amphibians” A male Crowned Tree Frog (Anotheca spinosa) peering out from a tree hole. 2 Text by: Brian Kubicki Photography by: Brian Kubicki Version: 3.1 (October 12th, 2009) Mailing Address: Apdo. 81-7200, Siquirres, Provincia de Limón, Costa Rica Telephone: (506)-8889-0655, (506)-8841-5327 Web: www.cramphibian.com Email: [email protected] Cover Photo: Mountain Glass Frog (Sachatamia ilex), Quebrada Monge, C.R.A.R.C. Reserve. 3 Costa Rica is internationally recognized as one of the most biologically diverse countries on the planet in total species numbers for many taxonomic groups of flora and fauna, one of those being amphibians. Costa Rica has 190 species of amphibians known from within its tiny 51,032 square kilometers territory. With 3.72 amphibian species per 1,000 sq. km. of national territory, Costa Rica is one of the richest countries in the world regarding amphibian diversity density. Amphibians are under constant threat by contamination, deforestation, climatic change, and disease. The majority of Costa Rica’s amphibians are surrounded by mystery in regards to their basic biology and roles in the ecology. Through intense research in the natural environment and in captivity many important aspects of their biology and conservation can become better known. The Costa Rican Amphibian Research Center (C.R.A.R.C.) was established in 2002, and is a privately owned and operated conservational and biological research center dedicated to studying, understanding, and conserving one of the most ecologically important animal groups of Neotropical humid forest ecosystems, that of the amphibians. -

Missouri's Toads and Frogs Booklet
TOADSMissouri’s andFROGS by Jeffrey T. Briggler and Tom R. Johnson, Herpetologists www.MissouriConservation.org © 1982, 2008 Missouri Conservation Commission Equal opportunity to participate in and benefit from programs of the Missouri Department of Conservation is available to all individuals without regard to their race, color, national origin, sex, age or disability. Questions should be directed to the Department of Conservation, P.O. Box 180, Jefferson City, MO 65102, (573) 751-4115 (voice) or 800-735-2966 (TTY), or to the U.S. Fish and Wildlife Service Division of Federal Assistance, 4401 N. Fairfax Drive, Mail Stop: MBSP-4020, Arlington, VA 22203. Cover photo: Eastern gray treefrog by Tom R. Johnson issouri toads and frogs are colorful, harmless, vocal and valuable. Our forests, prairies, rivers, swamps and marshes are Mhome to a multitude of toads and frogs, but few people know how many varieties we have, how to tell them apart, or much about their natural history. Studying these animals and sharing their stories with fellow Missourians is one of the most pleasurable and rewarding aspects of our work. Toads and frogs are amphibians—a class Like most of vertebrate animals that also includes amphibians, salamanders and the tropical caecilians, which are long, slender, wormlike and legless. frogs and Missouri has 26 species and subspecies (or toads have geographic races) of toads and frogs. Toads and frogs differ from salamanders by having an aquatic relatively short bodies and lacking tails at adulthood. Being an amphibian means that tadpole stage they live two lives: an aquatic larval or tadpole and a semi- stage and a semi-aquatic or terrestrial adult stage. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species. -

Common FROGS of La Selva Biological Station, Costa Rica
Common FROGS of La Selva Biological Station, Costa Rica 1 2 3 3 1 1 Michelle E. Thompson , Juan G. Abarca , Enrique Salicetti Nelson , Hansell Rodriguez Vega, Carlos de la Rosa , Ralph Garcia Robleto, Maureen A. Donnelly 1Florida International University, 2Universidad de Costa Rica, 3Organization for Tropical Studies Photos: Juan G. Abarca (JGA), Carlos de la Rosa (CdlR), Ralph Garcia Robleto (RGR), Hansell Rodriguez Vega (HRV), Enrique Salicetti Nelson (ESN), Michelle E. Thompson (MET) Produced by: Michelle E. Thompson. Thanks to: José Iván Castillo Gomez and Selva Verde Lodge © Michelle E. Thompson [[email protected]] (M) Male, (Juv.) Juvenile; Lengths (Savage 2002) [fieldguides.fieldmuseum.org] [860] version 1 02/2017 1 Incilius melanochlorus 2 Rhaebo haematiticus 3 Rhinella marina 4 Espadarana prosoblepon (M) <104mm photo: ESN <81mm photo: CdlR <176mm photo: MET <32mm photo: MET BUFONIDAE BUFONIDAE BUFONIDAE CENTROLENIDAE 5 Hyalinobatrachium fleischmanni 6 Hyalinobatrachium valerioi 7 Teratohyla spinosa 8 Craugastor bransfordii <32mm photo: MET <27mm photo: ESN <24mm photo: JGA <27mm photo: ESN CENTROLENIDAE CENTROLENIDAE CENTROLENIDAE CRAUGASTORIDAE 9 Craugastor crassidigitus 10 Craugastor fitzingeri 11 Craugastor fitzingeri 12 Craugastor megacephalus <27mm photo: HRV <54mm photo: MET <54mm photo: MET <71mm photo: MET CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE 13 Craugastor mimus 14 Craugastor noblei 15 Craugastor talamancae 16 Diasporus diastema <59mm photo: JGA <67mm photo: MET <51mm photo: JGA <25mm photo: JGA CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE 17 Pristimantis cerasinus 18 Pristimantis cruentus 19 Pristimantis ridens 20 Dendrobates auratus <36mm photo: HRV <43mm photo: MET <26mm photo: ESN <43mm photo: MET CRAUGASTORIDAE CRAUGASTORIDAE CRAUGASTORIDAE DENDROBATIDAE Common FROGS of La Selva Biological Station, Costa Rica 1 2 3 3 1 2 Michelle E. -

The Relative Impacts of Native and Introduced Predatory Fish on a Temporary Wetland Tadpole Assemblage
Oecologia (2003) 136:289–295 DOI 10.1007/s00442-003-1251-2 COMMUNITY ECOLOGY Matthew J. Baber · Kimberly J. Babbitt The relative impacts of native and introduced predatory fish on a temporary wetland tadpole assemblage Received: 18 October 2002 / Accepted: 7 March 2003 / Published online: 24 April 2003 Springer-Verlag 2003 Abstract Understanding the relative impacts of predators tor-prey encounter rates, and thus predation rate. In on prey may improve the ability to predict the effects of combination with a related field study, our results suggest predator composition changes on prey assemblages. We that native predatory fish play a stronger role than C. experimentally examined the relative impact of native and batrachus in influencing the spatial distribution and introduced predatory fish on a temporary wetland am- abundance of temporary wetland amphibians in the phibian assemblage to determine whether these predators landscape. exert distinct (unique or non-substitutable) or equivalent (similar) impacts on prey. Predatory fish included the Keywords Fish assemblages · Functional relationships · eastern mosquitofish (Gambusia holbrooki), golden top- Larval anurans · Predation · Temporary wetlands minnow (Fundulus chrysotus), flagfish (Jordanella flori- dae), and the introduced walking catfish (Clarias batrachus). The tadpole assemblage included four com- Introduction mon species known to co-occur in temporary wetlands in south-central Florida, USA: the oak toad (Bufo querci- Predators that occupy similar trophic positions may have cus), pinewoods treefrog (Hyla femoralis), squirrel distinct or equivalent effects on prey community dynam- treefrog (Hyla squirella), and eastern narrowmouth toad ics (Lawton and Brown 1993; Morin 1995; Kurzava and (Gastrophryne carolinensis). Tadpoles were exposed to Morin 1998). -

Biology of Chordates Video Guide
Branches on the Tree of Life DVD – CHORDATES Written and photographed by David Denning and Bruce Russell ©2005, BioMEDIA ASSOCIATES (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE DVD PROGRAM) .. .. To many students, the phylum Chordata doesn’t seem to make much sense. It contains such apparently disparate animals as tunicates (sea squirts), lancelets, fish and humans. This program explores the evolution, structure and classification of chordates with the main goal to clarify the unity of Phylum Chordata. All chordates possess four characteristics that define the phylum, although in most species, these characteristics can only be seen during a relatively small portion of the life cycle (and this is often an embryonic or larval stage, when the animal is difficult to observe). These defining characteristics are: the notochord (dorsal stiffening rod), a hollow dorsal nerve cord; pharyngeal gills; and a post anal tail that includes the notochord and nerve cord. Subphylum Urochordata The most primitive chordates are the tunicates or sea squirts, and closely related groups such as the larvaceans (Appendicularians). In tunicates, the chordate characteristics can be observed only by examining the entire life cycle. The adult feeds using a ‘pharyngeal basket’, a type of pharyngeal gill formed into a mesh-like basket. Cilia on the gill draw water into the mouth, through the basket mesh and out the excurrent siphon. Tunicates have an unusual heart which pumps by ‘wringing out’. It also reverses direction periodically. Tunicates are usually hermaphroditic, often casting eggs and sperm directly into the sea. After fertilization, the zygote develops into a ‘tadpole larva’. This swimming larva shows the remaining three chordate characters - notochord, dorsal nerve cord and post-anal tail.