2018, Diatom Assemblage in the 24 Cm Upper Sediment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crater Lakes of Java: Dieng, Kelud and Ijen
Crater lakes of Java: Dieng, Kelud and Ijen Excursion Guidebook IAVCEI General Assembly, Bali 2000 Manfred J. van Bergen1, Alain Bernard2, Sri Sumarti3, Terry Sriwana3 and Kastiman Sitorus3 1Faculty of Earth Sciences, Utrecht University, Budapestlaan 4, 3584 CD Utrecht, the Netherlands; 2BRUEGEL (Brussels Unit for Environmental, Geochemical and Life Sciences Studies), Université Libre de Bruxelles 160/02, 50 Ave. Roosevelt, 1050 Brussels, Belgium; 3Volcanological Survey of Indonesia, Jl. Diponegoro 57, Bandung 40122, and Jalan Cendana 15, Yogyakarta 55166, Indonesia. June, 2000 Dieng Plateau Java Die ng Kelud Ijen The Dieng Volcanic Complex in Central Java is situated on a highland plateau at about 2000 m above sea level, approximately 25 km north of the city of Wonosobo. It belongs to a series of Quaternary volcanoes, which includes the historically active Sumbing and Sundoro volcanoes. The plateau is a rich agricultural area for potatoes, cabbages, tomatoes and other vegetables. There are numerous surface manifestations of hydrothermal activity, including lakes, fumaroles/solfatara and hotsprings. The area is also known for the development of geothermal resources and lethal outbursts of gas. Scattered temples are the witnesses of the ancient Hindu culture that once reigned. Geological setting The 14 km long and 6 km wide Dieng Plateau has a general E-W trend due to the shift of eruptive centers with the youngest activity being in the east. It is underlain by Tertiary marls, limestones, tuffaceous sandstones and volcanics. The Dieng Complex itself consists of late Quaternary to Recent volcanic cones and explosion craters, formed at the intersection of two major fault zones trending E-W and NW-SE. -
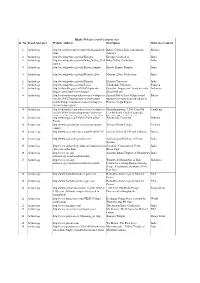
2.Hindu Websites Sorted Category Wise
Hindu Websites sorted Category wise Sl. No. Broad catergory Website Address Description Reference Country 1 Archaelogy http://aryaculture.tripod.com/vedicdharma/id10. India's Cultural Link with Ancient Mexico html America 2 Archaelogy http://en.wikipedia.org/wiki/Harappa Harappa Civilisation India 3 Archaelogy http://en.wikipedia.org/wiki/Indus_Valley_Civil Indus Valley Civilisation India ization 4 Archaelogy http://en.wikipedia.org/wiki/Kiradu_temples Kiradu Barmer Temples India 5 Archaelogy http://en.wikipedia.org/wiki/Mohenjo_Daro Mohenjo_Daro Civilisation India 6 Archaelogy http://en.wikipedia.org/wiki/Nalanda Nalanda University India 7 Archaelogy http://en.wikipedia.org/wiki/Taxila Takshashila University Pakistan 8 Archaelogy http://selians.blogspot.in/2010/01/ganesha- Ganesha, ‘lingga yoni’ found at newly Indonesia lingga-yoni-found-at-newly.html discovered site 9 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Ancient Idol of Lord Vishnu found Russia om/2012/05/27/ancient-idol-of-lord-vishnu- during excavation in an old village in found-during-excavation-in-an-old-village-in- Russia’s Volga Region russias-volga-region/ 10 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Mahendraparvata, 1,200-Year-Old Cambodia om/2013/06/15/mahendraparvata-1200-year- Lost Medieval City In Cambodia, old-lost-medieval-city-in-cambodia-unearthed- Unearthed By Archaeologists 11 Archaelogy http://wikimapia.org/7359843/Takshashila- Takshashila University Pakistan Taxila 12 Archaelogy http://www.agamahindu.com/vietnam-hindu- Vietnam -

The Liangan Temple Site in Central Java Novida Abbas (Ed.) (2016), Liangan
Archipel Études interdisciplinaires sur le monde insulindien 94 | 2017 Varia The Liangan Temple Site in Central Java Novida Abbas (ed.) (2016), Liangan. Mozaik Peradaban Mataram Kuno di Lereng Sindoro. Second Edition. Yogyakarta: Kepel Press. xi + 357 p., bibliographie. ISBN 978-602-1228-72-2 Véronique Degroot Electronic version URL: https://journals.openedition.org/archipel/456 DOI: 10.4000/archipel.456 ISSN: 2104-3655 Publisher Association Archipel Printed version Date of publication: 6 December 2017 Number of pages: 191-209 ISBN: 978-2-910513-78-8 ISSN: 0044-8613 Electronic reference Véronique Degroot, “The Liangan Temple Site in Central Java”, Archipel [Online], 94 | 2017, Online since 06 December 2017, connection on 27 August 2021. URL: http://journals.openedition.org/archipel/456 ; DOI: https://doi.org/10.4000/archipel.456 Association Archipel À PROPOS DE VÉRONIQUE DEGROOT1 The Liangan Temple Site in Central Java Novida Abbas (ed.) (2016), Liangan. Mozaik Peradaban Mataram Kuno di Lereng Sindoro. Second Edition. Yogyakarta: Kepel Press. xi + 357 p., biblio- graphie. ISBN 978-602-1228-72-2 1Candi Liangan was accidentally discovered in 2008 by inhabitants of the nearby village of Liangan, Temanggung, Central Java. The site was buried beneath meters of volcanic debris deposited by lahars, pyroclastic flows and ash falls. Organic materials had been burnt but at the same time the site had been sealed and preserved, waiting for archaeologists to unearth it. It is thus no wonder that Candi Liangan has yielded a wide range of archaeological material, from earthenware to plant remains and in situ wooden structures. Because of its exceptional state of preservation, Candi Liangan provides a unique perspective on the life of a religious community of 9th-century Central Java. -

Early Connections
Deakin Research Online This is the published version: Datta, Sambit and Beynon, David 2011, Early Connections : reflections on the canonical lineage of Southeast Asian temples, in EAAC 2011 : South of East Asia : Re-addressing East Asian Architecture and Urbanism : Proceedings of the East Asian Architectural Culture International Conference, Department of Architecture, National University of Singapore, Singapore, pp. 1-17. Available from Deakin Research Online: http://hdl.handle.net/10536/DRO/DU:30045633 Every reasonable effort has been made to ensure that permission has been obtained for items included in Deakin Research Online. If you believe that your rights have been infringed by this repository, please contact [email protected] Copyright : 2011, National University of Singapore Early Connections: Reflections on the canonical lineage of Southeast Asian temples Sambit Datta1, David Beynon2 1 School of Built Environment, Curtin University 2 School of Architecture and Building, Deakin University Abstract Temples were constructed across Southeast Asia following the spread of Brahmanic/Hindu culture between the fifth to eight centuries CE. Epigraphic evidence, architectural and stylistic similarities between temples in the region are strongly indicative of historic cross cultural links between the traditions. This paper presents the findings of a research project that pieces together fragments of evidence from early temple sites in Southeast Asia to establish the linkages between the Southeast Asian temple building traditions. The focus of the paper is on tracing the canonical connections between these traditions through an examination of temple sites in Cambodia and Java respectively. The legacy of this ancient diasporic movement remains celebrated today in the admiration of Southeast Asian monuments such as Angkor Wat and Prambanan. -

Morphological Typology and Origins of the Hindu-Buddhist Candis Which Were Built from 8Th to 17Th Centuries in the Island of Bali
計画系 642 号 【カテゴリーⅠ】 日本建築学会計画系論文集 第74巻 第642号,1857-1866,2009年 8 月 J. Archit. Plann., AIJ, Vol. 74 No. 642, 1857-1866, Aug., 2009 MORPHOLOGICAL TYPOLOGY AND ORIGINS OF THE MORPHOLOGICALHINDU-BUDDHIST TYPOLOGY CANDI ANDARCHITECTURE ORIGINS OF THE HINDU-BUDDHIST CANDI ARCHITECTURE IN BALI ISLAND IN BALI ISLAND バリ島におけるヒンドゥー・仏教チャンディ建築の起源と類型に関する形態学的研究 �������������������������������������� *1 *2 *3 I WayanI Wayan KASTAWAN KASTAWAN * ,¹, Yasuyuki Yasuyuki NAGAFUCHINAGAFUCHI * ² and and Kazuyoshi Kazuyoshi FUMOTO FUMOTO * ³ イ �ワヤン ��� カスタワン ��������,永 渕 康���� 之,麓 �� 和 善 This paper attempts to investigate and analyze the morphological typology and origins of the Hindu-Buddhist candis which were built from 8th to 17th centuries in the island of Bali. Mainly, the discussion will be focused on its characteristics analysis and morphology in order to determine the candi typology in its successive historical period, and the origin will be decided by tracing and comparative study to the other candis that are located across over the island and country as well. As a result, 2 groups which consist of 6 types of `Classical Period` and 1 type as a transition type to `Later Balinese Period`. Then, the Balinese candis can also be categorized into the `Main Type Group` which consists of 3 types, such as Stupa, Prasada, Meru and the `Complementary Type Group` can be divided into 4 types, like Petirthan, Gua, ������ and Gapura. Each type might be divided into 1, 2 or 3 sub-types within its architectural variations. Finally, it is not only the similarities of their candi characteristics and typology can be found but also there were some influences on the development of candis in the Bali Island that originally came from Central and East Java. -

Candi, Space and Landscape
Degroot Candi, Space and Landscape A study on the distribution, orientation and spatial Candi, Space and Landscape organization of Central Javanese temple remains Central Javanese temples were not built anywhere and anyhow. On the con- trary: their positions within the landscape and their architectural designs were determined by socio-cultural, religious and economic factors. This book ex- plores the correlations between temple distribution, natural surroundings and architectural design to understand how Central Javanese people structured Candi, Space and Landscape the space around them, and how the religious landscape thus created devel- oped. Besides questions related to territory and landscape, this book analyzes the structure of the built space and its possible relations with conceptualized space, showing the influence of imported Indian concepts, as well as their limits. Going off the beaten track, the present study explores the hundreds of small sites that scatter the landscape of Central Java. It is also one of very few stud- ies to apply the methods of spatial archaeology to Central Javanese temples and the first in almost one century to present a descriptive inventory of the remains of this region. ISBN 978-90-8890-039-6 Sidestone Sidestone Press Véronique Degroot ISBN: 978-90-8890-039-6 Bestelnummer: SSP55960001 69396557 9 789088 900396 Sidestone Press / RMV 3 8 Mededelingen van het Rijksmuseum voor Volkenkunde, Leiden CANDI, SPACE AND LANDscAPE Sidestone Press Thesis submitted on the 6th of May 2009 for the degree of Doctor of Philosophy, Leiden University. Supervisors: Prof. dr. B. Arps and Prof. dr. M.J. Klokke Referee: Prof. dr. J. Miksic Mededelingen van het Rijksmuseum voor Volkenkunde No. -

Trace Metals and Diatom Stratigraphy Along the Sill Between Lakes Telaga Warna and Telaga Pengilon, Dieng, Central Java, Indonesia
sustainability Article Trace Metals and Diatom Stratigraphy along the Sill between Lakes Telaga Warna and Telaga Pengilon, Dieng, Central Java, Indonesia Kenanga Sari 1,2, Tri Retnaningsih Soeprobowati 1,2,3,* , Jumari Jumari 1,2, Riche Hariyati 1,2 and Jerry R. Miller 4 1 Center for Paleolimnoloy (CPalim), Universitas Diponegoro, Semarang 50241, Indonesia; [email protected] (K.S.); [email protected] (J.J.); [email protected] (R.H.) 2 Department Biology, Faculty of Science and Mathematics, Universitas Diponegoro, Semarang 50275, Indonesia 3 School of Postgraduate Studies, Universitas Diponegoro, Semarang 50241, Indonesia 4 Department of Geosciences and Natural Resources, Western Carolina University, Cullowhee, NC 28723, USA; [email protected] * Correspondence: [email protected] Abstract: This study examines the spatiotemporal variations in diatom assemblages and selected metal concentrations (Pb, Cr, Cd, Al, and Zn) in bed sediments of lakes Telaga Pengilon and Telaga Warna in Dieng, Indonesia to document natural and/or anthropogenic changes in the local aquatic and terrestrial environment. The analyses focused on sediments collected from a 150-cm core taken from a sill between the two lakes, which exhibit significant differences in water chemistry. The core was subdivided into 14 stratigraphic intervals allowing for an analysis of the vertical (and temporal) variations in diatom composition and selected metal concentrations. A total of 103 taxa from 25 genera were identified in the core. Diatom assemblages were dominated by Eunotia (56%), Citation: Sari, K.; Soeprobowati, T.R.; Pinnularia (17.2%), and Frustulia (4.6%). The most abundant species was Eunotia, a diatom that Jumari, J.; Hariyati, R.; Miller, J.R. -

Filling the Gaps - an Action Plan for the Future
The World Heritage List: Filling the Gaps - an Action Plan for the Future An Analysis by ICOMOS February 2004 49-51 rue de la Fédération - 75015 Paris - France - Tel + 33 1 45 67 67 70 - Fax + 33 1 45 66 06 22 Executive Summary The Scope of the Analysis This ICOMOS analysis on the World Heritage List and Tentative Lists should be seen as a contribution to the further development of the Global Strategy for a credible, representative and balanced World Heritage List. This analysis is a response to the invitation by the World Heritage Committee at its 24th Session in Cairns (2000) to: “proceed with an analysis of sites inscribed on the World Heritage List and the Tentative List on a regional, chronological, geographical and thematic basis”. The proposed scope of the analysis was to “provide States Parties with a clear overview of the present situation, and likely trends in the short- to medium- term with a view to identifying under-represented categories”. Organisation of the Analysis The ICOMOS analysis has been based on three complementary approaches to the analysis of the representivity of the World Heritage List: A. Typological Framework based on categories B. Chronological-Regional Framework C. Thematic Framework The study was carried out in two phases: the first phase was undertaken by Henry Cleere in 2002 and early 2003. It focused on a typological analysis of the World Heritage List and Tentative Lists and it included two meetings of an international working group, in Paris, France (March 2002) and Zaragoza, Spain (December 2002). The second phase was carried out by an ICOMOS team coordinated by Michael Petzet in the second half of 2003 and in early 2004. -

RESEARCH OPEN ACCESS Interpretation of Symbols
Surpi et al. Space and Culture, India 2021, 8:4 Page | 60 https://doi.org/10.20896/saci.v8i4.991 RESEARCH OPEN ACCESS Interpretation of Symbols, Veneration and Divine Attributes in Dieng Temple Complex, Central Java Ni Kadek Surpi,†* Ni Nyoman Ayu Nikki Avalokitesvari,Ì I Made Gami Sandi UntaraÎ and I Ketut Sudarsana† Abstract This study aims to discuss the divine symbols and attributes used as a medium of worship in the Dieng Plateau. The research was phased in according to Wallace's empirical cycle and was conducted in the Dieng Plateau, Central Java, Indonesia, a spiritual centre in ancient Java. The discovery of the Śiva Triśirah statue in the Dieng Temple Complex reveals new things in the past Hindu Nusantara Theology construction. Several divine symbols and attributes are served as a medium of worship at the Temple Complex in the Dieng Plateau. The concept of Deity in the Dieng Plateau is Śivaistic in character with the worship of Lord Śiva Triśirah, that is, Śiva with three faces and four hands, as the Supreme Deity. However, some divine symbols and attributes also serve as a medium of worship and connected to divinity. In Hinduism, the sacred symbols and attributes of God are inseparable. Divine attributes generally define God. In the discussion of theology, God is described with various excellent attributes. The central divine attributes found are as follows: Omnipotence, Creatorship, Omniscience, Eternity and Omnipresence, Personhood, Goodness⁄ Perfection, Non-Physicality, Necessary, Existence, Simplicity, Immutability and Impassibility. These divine attributes are depicted in various forms of sacred symbols found in the Dieng Plateau. -

David Beynon and Sambit Datta
Proceedings of the Society of Architectural Historians, Australia and New Zealand 30, Open Papers presented to the 30th Annual Conference of the Society of Architectural Historians, Australia and New Zealand held on the Gold Coast, Queensland, Australia, July 2-5, 2013. http://www.griffith.edu.au/conference/sahanz-2013/ David Beynon and Sambit Datta, “The Construction Geometry of Early Javanese Temples” in Proceedings of the Society of Architectural Historians, Australia and New Zealand: 30, Open, edited by Alexandra Brown and Andrew Leach (Gold Coast, Qld: SAHANZ, 2013), vol. 1, p 327-340. ISBN-10: 0-9876055-0-X ISBN-13: 978-0-9876055-0-4 The Construction Geometry of Early Javanese Temples David Beynon, Deakin University Sambit Datta, Curtin University The early development of Hindu Javanese architecture can be traced through interpretation of epigraphs, archaeological excavations, and comparison of extant temples with other traditions. However, while many scholars have speculated on connections between Javanese Hindu temples and presumed antecedents in India, these have been made on the basis of visual comparison and epigraphic interpretations. No Indian temple has been conclusively shown to be a model for the earliest Javanese temples. Archaeologist and temple historian Michael Meister has shown in his analysis of the geometric composition of early Hindu temples in South Asia how a ritual sixty-four square mandala was the geometric basis of temple construction during the formative period (fifth to eighth century) of the Indian architectural tradition. Working from an understanding of temple construction sequence as well as their ritual underpinnings, Meister found that the sixty- four square mandala’s dimensions correlate closely to the constructed dimensions at the level of the vedibandha (which corresponds with the plan level of the sanctuary threshold). -

Candi Space and Landscape: a Study on the Distribution, Orientation and Spatial Organization of Central Javanese Temple Remains
Candi Space and Landscape: A Study on the Distribution, Orientation and Spatial Organization of Central Javanese Temple Remains Proefschrift ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van Rector Magnificus Prof. mr. P.F. van der Heijden, volgens besluit van het College voor Promoties te verdedigen op woensdag 6 mei 2009 klokke 13.45 uur door Véronique Myriam Yvonne Degroot geboren te Charleroi (België) in 1972 Promotiecommissie: Promotor: Prof. dr. B. Arps Co-promotor: Dr. M.J. Klokke Referent: Dr. J. Miksic, National University of Singapore. Overige leden: Prof. dr. C.L. Hofman Prof. dr. A. Griffiths, École Française d’Extrême-Orient, Paris. Prof. dr. J.A. Silk The realisation of this thesis was supported and enabled by the Netherlands Organisation for Scientific Research (NWO), the Gonda Foundation (KNAW) and the Research School of Asian, African and Amerindian Studies (CNWS), Leiden University. Acknowledgements My wish to research the relationship between Ancient Javanese architecture and its natural environment is probably born in 1993. That summer, I made a trip to Indonesia to complete the writing of my BA dissertation. There, on the upper slopes of the ever-clouded Ungaran volcano, looking at the sulfurous spring that runs between the shrines of Gedong Songo, I experienced the genius loci of Central Javanese architects. After my BA, I did many things and had many jobs, not all of them being archaeology-related. Nevertheless, when I finally arrived in Leiden to enroll as a PhD student, the subject naturally imposed itself upon me. Here is the result, a thesis exploring the notion of space in ancient Central Java, from the lay-out of the temple plan to the interrelationship between built and natural landscape. -

R. Jordaan the Sailendras, the Status of the Ksatriya Theory, and the Development of Hindu-Javanese Temple Architecture
R. Jordaan The Sailendras, the status of the Ksatriya theory, and the development of Hindu-Javanese temple architecture In: Bijdragen tot de Taal-, Land- en Volkenkunde 155 (1999), no: 2, Leiden, 210-243 This PDF-file was downloaded from http://www.kitlv-journals.nl Downloaded from Brill.com09/30/2021 09:26:13PM via free access ROY E. JORDAAN The Sailendras, the Status of the Ksatriya Theory, and the Development of Hindu- Javanese Temple Architecture Introduction The reconstruction of the history of Java, Sumatra, and the Malay Peninsula from the eighth to the twelfth century AD1 is still greatly hampered by the unsolved problem of the origin of the Sailendra dynasty. The reference to the name Sailendra in a number of dated Central Javanese inscriptions (Kalasan 778, Kelurak 782), an undated inscription found in the Thai part of the Malay Peninsula (Ligor B, in or shortly after 775), and a few inscriptions from both North and South India (Nalanda circa 859, Charter of Leyden 1089-1090) inspired R.C. Majumdar (1933, 1934) to posit the existence of a Sailendra empire, which, at the height of its glory, in the early ninth century, suppos- edly embraced large parts of the Indo-Malay archipelago (the Malay Penin- sula, Sumatra, and parts of Java) as well as of the Southeast Asian mainland. Majumdar suggested that the Sailendras originated from Kaliriga in India and, travelling by way of Lower Burma, migrated to the northern part of the Malay Peninsula, whence they ruled over their new kingdom, which he des- ignated 'Malayasia'. In the ensuing scholarly debate, various of Majumdar's ideas were dis- puted.