Full Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Relationships of the Starlings (Sturnidae: Sturnini) and the Mockingbirds (Sturnidae: Mimini)
THE RELATIONSHIPS OF THE STARLINGS (STURNIDAE: STURNINI) AND THE MOCKINGBIRDS (STURNIDAE: MIMINI) CHARLESG. SIBLEYAND JON E. AHLQUIST Departmentof Biologyand PeabodyMuseum of Natural History,Yale University, New Haven, Connecticut 06511 USA ABSTRACT.--OldWorld starlingshave been thought to be related to crowsand their allies, to weaverbirds, or to New World troupials. New World mockingbirdsand thrashershave usually been placed near the thrushesand/or wrens. DNA-DNA hybridization data indi- cated that starlingsand mockingbirdsare more closelyrelated to each other than either is to any other living taxon. Some avian systematistsdoubted this conclusion.Therefore, a more extensiveDNA hybridizationstudy was conducted,and a successfulsearch was made for other evidence of the relationshipbetween starlingsand mockingbirds.The resultssup- port our original conclusionthat the two groupsdiverged from a commonancestor in the late Oligoceneor early Miocene, about 23-28 million yearsago, and that their relationship may be expressedin our passerineclassification, based on DNA comparisons,by placing them as sistertribes in the Family Sturnidae,Superfamily Turdoidea, Parvorder Muscicapae, Suborder Passeres.Their next nearest relatives are the members of the Turdidae, including the typical thrushes,erithacine chats,and muscicapineflycatchers. Received 15 March 1983, acceptedI November1983. STARLINGS are confined to the Old World, dine thrushesinclude Turdus,Catharus, Hylocich- mockingbirdsand thrashersto the New World. la, Zootheraand Myadestes.d) Cinclusis -

Jungle Myna (Acridotheres Fuscus)
Invasive animal risk assessment Biosecurity Queensland Agriculture Fisheries and Department of Jungle myna Acridotheres fuscus Steve Csurhes First published 2011 Updated 2016 © State of Queensland, 2016. The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence visit http://creativecommons.org/licenses/by/3.0/au/ deed.en" http://creativecommons.org/licenses/by/3.0/au/deed.en Front cover: Jungle myna Photo: Used with permission, Wikimedia Commons. Invasive animal risk assessment: Jungle myna Acridotheres fuscus 2 Contents Summary 4 Introduction 5 Identity and taxonomy 5 Description and biology 5 Diet 5 Reproduction 5 Preferred habitat and climate 6 Native range and global distribution 6 Current distribution and impact in Queensland 6 History as a pest overseas 7 Use 7 Potential distribution and impact in Queensland 7 References 8 Invasive animal risk assessment: Jungle myna Acridotheres fuscus 3 Summary Acridotheres fuscus (jungle myna) is native to an extensive area of India and parts of southeast Asia. Naturalised populations exist in Singapore, Taiwan, Fiji, Western Samoa and elsewhere. In Fiji, the species occasionally causes significant damage to crops of ground nuts, with crop losses of up to 40% recorded. Within its native range (South India), it is not a well documented pest, but occasionally causes considerable (localised) damage to fruit orchards. -

British Lichen Society Bulletin No
1 BRITISH LICHEN SOCIETY OFFICERS AND CONTACTS 2010 PRESIDENT S.D. Ward, 14 Green Road, Ballyvaghan, Co. Clare, Ireland, email [email protected]. VICE-PRESIDENT B.P. Hilton, Beauregard, 5 Alscott Gardens, Alverdiscott, Barnstaple, Devon EX31 3QJ; e-mail [email protected] SECRETARY C. Ellis, Royal Botanic Garden, 20A Inverleith Row, Edinburgh EH3 5LR; email [email protected] TREASURER J.F. Skinner, 28 Parkanaur Avenue, Southend-on-Sea, Essex SS1 3HY, email [email protected] ASSISTANT TREASURER AND MEMBERSHIP SECRETARY H. Döring, Mycology Section, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, email [email protected] REGIONAL TREASURER (Americas) J.W. Hinds, 254 Forest Avenue, Orono, Maine 04473-3202, USA; email [email protected]. CHAIR OF THE DATA COMMITTEE D.J. Hill, Yew Tree Cottage, Yew Tree Lane, Compton Martin, Bristol BS40 6JS, email [email protected] MAPPING RECORDER AND ARCHIVIST M.R.D. Seaward, Department of Archaeological, Geographical & Environmental Sciences, University of Bradford, West Yorkshire BD7 1DP, email [email protected] DATA MANAGER J. Simkin, 41 North Road, Ponteland, Newcastle upon Tyne NE20 9UN, email [email protected] SENIOR EDITOR (LICHENOLOGIST) P.D. Crittenden, School of Life Science, The University, Nottingham NG7 2RD, email [email protected] BULLETIN EDITOR P.F. Cannon, CABI and Royal Botanic Gardens Kew; postal address Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, email [email protected] CHAIR OF CONSERVATION COMMITTEE & CONSERVATION OFFICER B.W. Edwards, DERC, Library Headquarters, Colliton Park, Dorchester, Dorset DT1 1XJ, email [email protected] CHAIR OF THE EDUCATION AND PROMOTION COMMITTEE: position currently vacant. -

Conservation of Breeding Colonies of the Bank Myna, Acridotheres Ginginianus (Latham 1790), in Chapai Nawabganj, Bangladesh
Univ. j. zool. Rajshahi Univ. Vol. 30, 2011 pp. 49-53 ISSN 1023-6104 http://journals.sfu.ca/bd/index.php/UJZRU © Rajshahi University Zoological Society Conservation of breeding colonies of the bank myna, Acridotheres ginginianus (Latham 1790), in Chapai Nawabganj, Bangladesh M Farid Ahsan and M Tarik Kabir Department of Zoology, University of Chittagong, Chittagong, Bangladesh Abstact: A study on the conservation on breeding colonies of bank myna, Acridotheres ginginianus (Latham 1790), was conducted in Chapai Nawabganj district of Bangladesh during a period from June 2007 to October 2009. The total population in the breeding colonies was estimated as 4,452. The preferable breeding habitats of bird are bank of the river, soil heap/ditches of brickfield and holes of culvert. Environmental calamities such as bank erosion and flooding, drought and rain affect the breeding colonies. Human settlement near breeding habitats, hunting, collecting eggs and nestlings by children, and use of nets to prevent nesting at bank of the river are the main human impacts on the breeding colonies. Major threats are flood, children-fun, predators and human for its meat. Conservation awareness for this species have depicted in this paper. Key words: Conservation, breeding colonies, bank myna, Acridotheres ginginianus, Chapai Nawabganj, Bangladesh. example, Grimmett et al. (1998), Inskipp et al. Introduction (1996), Sibley & Monroe (1993, 1990), Sibley & Conservation biology is the new multidisciplinary Ahlquist (1990), Ripley (1982), Husain (2003, science that -
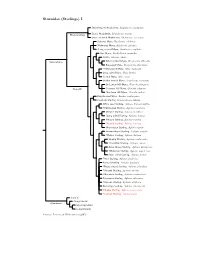
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

Chordate Sections
Utinomi's Bibliographica Micronesica: Chordate Sections HARVEY I. FISHER1 A COpy OF Bibliographica Micronesica / branches of science it would be inadvisable Scientiae Nattlraliset Cultus, by Dr. Huzio to start a study without some knowledge of Utinomi, became temporarily· available in the work carried on by Japanese scientists the Territory of Hawaii late in the summer in the mandated islands. of 1946. This bibliography of 208 pages Because of the above facts it seems desir was published in 1944 by the Hokuryiikan able to publish immediately all the titles Publishing Company in Tokyo. A negative given by Utino.(l1i, and to add translations microfilm was made by the University of of the titles and publications cited in the Hawaii Library, and later certain sections Japanese language. The present paper in were enlarged and printed photograph cludes only those sections dealing with chor ically. date animals, and constitutes pages 24 to 43 An interest in the vertebrate animals of of the original publication, in addition to the Micronesia, especially those of Yap, led me translated Preface and Explanatory Notes. to have certain Japanese titles translated for The list of titles is of course not exhaus personal use. It soon became evident that tive, but it is not the purpose of this pub although the bibliography was not com lication to. add titles to Utinomi's list. A plete, it did include many significant titles complete bibliography of the chordates in that had previously been overlooked by Micronesia would take years of preparation workers in vertebrate zoology. and research in many libraries. The imme This bibliography has great interest at the diate usefulness of the bibliography in its present time. -

Aplonis Opaca) by Brown Treesnakes (Boiga Irregularis) on Guam*
Micronesica 2018-06: 1–7 First reported predation of fledgling Micronesian starlings (Aplonis opaca) by brown treesnakes (Boiga irregularis) on Guam* CHRISTOPHER WAGNER†1, CAROLIN TAPPE1, MARTIN KASTNER1,2, OVIDIO JARAMILLO1,2, NOAH VAN EE3, JULIE SAVIDGE1 & HENRY S. POLLOCK1,2 1Department of Fish, Wildlife & Conservation Biology, Colorado State University, Fort Collins, CO 80523, USA 2Current address: School of Global Environmental Sustainability, Colorado State University, Fort Collins, CO 80523, USA 3Cherokee Nation Technologies, U.S. Geological Survey, Fort Collins Science Center, 2150 Centre Avenue, Building C, Fort Collins, CO 80526, USA Abstract— The brown treesnake’s (Boiga irregularis – BTS) inVasion of Guam is a primary example of the devastation an inVasive predator can haVe on island ecosystems. Its introduction following WWII caused the extirpation of most of the island’s aVifauna, although some native species have managed to persist in the presence of BTS, including a small population of Micronesian starlings (Aplonis opaca) on a military installation (Andersen Air Force Base – AAFB) in northeastern Guam. HoweVer, it remains unclear to what extent BTS predation continues to impact the population, particularly during the vulnerable post-fledging period. We report the first direct observations of BTS predation on Micronesian starling fledglings (n = 15). All 15 snakes that consumed starlings were in good to exceptional body condition and most were found in urban habitat within 100 m of the nest box of the depredated fledgling. Following the predation events, snakes tended to seek refugia and remain inactive for 4.0 ± 1.67 (mean ± SD) days. Our findings provide evidence of ongoing BTS predation of a native bird species in areas undergoing operational snake remoVal and indicate that current control efforts are not sufficient to preVent BTS from encroaching into urban areas on AAFB to access high quality prey resources such as birds and mammals. -

University Micrtifilms International 300 N
HAWAII - 1819 - 1830: YEARS OF DECISION Item Type text; Thesis-Reproduction (electronic) Authors Corley, Janetta Susan Williamson Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 25/09/2021 20:57:14 Link to Item http://hdl.handle.net/10150/291446 INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided \to help you understand markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. -

Ecology, Abundance and Distribution Assessment of the Endemic Rarotonga Starling (Aplonis Cinerascens)
Ecology, abundance and distribution assessment of the endemic Rarotonga Starling (Aplonis cinerascens) Ana Tiraa st109560 School of Environment, Resources and Development Natural Resources Management August 2010 Abstract Aplonis cinerascens, or I’oi, an endemic Starling found on the island of Rarotonga, Cook Islands, was studied through field observations, literature review, questionnaire surveys and communication with knowledgeable individuals and research institutions. The Rarotongan species represents the most southerly and easterly extent in the range of the extant Aplonis genus. Previous research on the species has been ad hoc in manner. This study is the first attempt in dedicating research on I’oi, and aims to consolidate all currently available information in to one report, together with results of an investigation of the distribution, abundance, and ecology of the bird. Key words: I’oi, Starling, Distribution, Abundance, Aplonis cinerascens, Rarotonga, Cook Islands, Ecology 1 Introduction The Cook Islands comprises 15 small islands scattered over 1.8 million square kilometres of the South Pacific Ocean. Located between latitudes 9 degrees and 22 degrees south and longitudes 157 degrees and 166 degrees West, the islands are flanked by Samoa and Tonga on the west, French Polynesia on the east, and Kiribati to the North. With a total land area of only 240 sq. km, the islands are divided geographically, into the Northern and Southern groups. Six islands make up the Northern group - Suwarrow, Nassau, Pukapuka, Rakahanga, Manihiki and Penryhn while the Southern group comprises nine islands - Palmerston, Aitutaki, Manuae, Takutea, Atiu, Mitiaro, Mauke, Mangaia and Rarotonga. Rarotonga is of volcanic origin, and is the commercial center of the Cook Islands. -

Voyages to Hawaii Before 1860
Voyages to Hawaii before 1860 Voyages to Hawaii before 1860 A Record, Based on Historical Narratives in the Libraries of the Hawaiian Mission Children’s Society and The Hawaiian Historical Society, Extended to March 1860 BERNICE JUDD enlarged and edited by HELEN YONGE LIND THE UNIVERSITY PRESS OF HAWAII for HAWAIIAN MISSION CHILDREN’S SOCIETY Honolulu Open Access edition funded by the National En- dowment for the Humanities / Andrew W. Mellon Foundation Humanities Open Book Program. Licensed under the terms of Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 In- ternational (CC BY-NC-ND 4.0), which permits readers to freely download and share the work in print or electronic format for non-commercial purposes, so long as credit is given to the au- thor. Derivative works and commercial uses require permission from the publisher. For details, see https://creativecommons.org/licenses/by-nc-nd/4.0/. The Cre- ative Commons license described above does not apply to any material that is separately copyrighted. Open Access ISBNs: 9780824883928 (PDF) 9780824883935 (EPUB) This version created: 5 September, 2019 Please visit www.hawaiiopen.org for more Open Access works from University of Hawai‘i Press. This edition is a revision of that originally published in 1929 by the Hawaiian Mission Children’s Society. Copyright © 1974 by The University Press of Hawaii All rights reserved IN MEMORY OF BERNICE JUDD The earlier edition of this book, published in 1929, was written by Bernice Judd. She kept two interleaved copies in which she noted further entries during her thirty-three years’ work in the Hawaiian Mission Children’s Society library. -

Singing Starlings Aplonis Cantoroides and Other Birds on Boigu Island, Torres Strait, Queensland
AUSTRALIAN 20 BIRD WATCHER AUSTRALIAN BIRD WATCHER 1997, 17, 20-24 Singing Starlings Aplonis cantoroides and Other Birds on Boigu Island, Torres Strait, Queensland by MIKE CARTER1, RORY O'BRIEN2 and NEIL MACUMBER3 130 Canadian Bay Road, Mt Eliza, Victoria 3930 23 Valentine Avenue, Kew, Victoria 3101 3C/- Post Office, Pomonal, Victoria 3381 Summary Ornithological reports from islands in Torres Strait are scarce. Birds seen during a visit to Boigu Island in July 1995 are listed. Significant sightings include Singing Starling Aplonis cantoroides (first authenticated record for Australia), Whiskered Tern Chlidonias hybridus, fig-parrot Cyclopsitta sp., Frilled Monarch Arses telescopthalmus, Northern Fantail Rhipidura rufiventris and House Sparrow Passer domesticus. Introduction Boigu Island is situated about 10 km south of the coast of Papua New Guinea (PNG) in Torres Strait, Queensland, at 9 °17 'S, 142 °13 'E (Draffan et al. 1983). It is the most northerly part of Australia. The island of 7150 ha has one small settlement, with most of the remainder forest and fresh-water swamp (O'Brien 1995). As a consequence of its proximity to PNG, its avifauna is more New Guinean than Australian. We visited Boigu from 18 to 20 July 1995, and here provide an annotated list of 60 species recorded (Appendix 1). All observations were within 3 km of the village. The swampy nature of the terrain makes access to most of the island difficult. We added 15 species to the 72 listed for the island by Draffan et al. (1983) in just two days, indicating the lack of ornithological reports from the island. -

Downloaded from on 27/06/2017
RIS for Site no. 2313, O Le Pupū Puē National Park, Samoa Ramsar Information Sheet Published on 6 October 2017 Samoa O Le Pupū Puē National Park Designation date 2 February 2016 Site number 2313 Coordinates 13°59'S 171°43'53"W Area 5 019,00 ha https://rsis.ramsar.org/ris/2313 Created by RSIS V.1.6 on - 18 May 2020 RIS for Site no. 2313, O Le Pupū Puē National Park, Samoa Color codes Fields back-shaded in light blue relate to data and information required only for RIS updates. Note that some fields concerning aspects of Part 3, the Ecological Character Description of the RIS (tinted in purple), are not expected to be completed as part of a standard RIS, but are included for completeness so as to provide the requested consistency between the RIS and the format of a ‘full’ Ecological Character Description, as adopted in Resolution X.15 (2008). If a Contracting Party does have information available that is relevant to these fields (for example from a national format Ecological Character Description) it may, if it wishes to, include information in these additional fields. 1 - Summary Summary O Le Pupū Puē National Park (OLPP) was established in 1978 as the first ever National Park in Samoa and the South Pacific region. It is located on the southern part of Upolu Island and extends from the highest points on the island (Mt. Vaivai, 1158 m), Mt. Fito (1120 m) and Mt. Puē (1020 m) down to the rugged Le Pupū lava coastal cliffs. The Site therefore has the full range of ecosystems from the littoral forests on the rugged coastal ridges, to the lowland rainforest, extending to the ridge rainforests along the watershed area to the montane forests.