A Field Guide to Diseases and Insect Pests of Northern and Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spruce Beetle
QUICK GUIDE SERIES FM 2014-1 Spruce Beetle An Agent of Subalpine Change The spruce beetle is a native species in Colorado’s spruce forest ecosystem. Endemic populations are always present, and epidemics are a natural part of the changing forest. There usually are long intervals between such events as insect and disease epidemics and wildfires, giving spruce forests time to regenerate. Prior to their occurrence, the potential impacts of these natural disturbances can be reduced through proactive forest management. The spruce beetle (Dendroctonus rufipennis) is responsible for the death of more spruce trees in North America than any other natural agent. Spruce beetle populations range from Alaska and Newfoundland to as far south as Arizona and New Mexico. The subalpine Engelmann spruce is the primary host tree, but the beetles will infest any Figure 1. Engelmann spruce trees infested spruce tree species within their geographical range, including blue spruce. In with spruce beetles on Spring Creek Pass. Colorado, the beetles are most commonly observed in high-elevation spruce Photo: William M. Ciesla forests above 9,000 feet. At endemic or low population levels, spruce beetles generally infest only downed trees. However, as spruce beetle population levels in downed trees increase, usually following an avalanche or windthrow event – a high-wind event that topples trees over a large area – the beetles also will infest live standing trees. Spruce beetles prefer large (16 inches in diameter or greater), mature and over- mature spruce trees in slow-growing, spruce-dominated stands. However, at epidemic levels, or when large-scale, rapid population increases occur, spruce beetles may attack trees as small as 3 inches in diameter. -

Seasonality and Lure Preference of Bark Beetles (Curculionidae: Scolytinae) and Associates in a Northern Arizona Ponderosa Pine Forest
COMMUNITY AND ECOSYSTEM ECOLOGY Seasonality and Lure Preference of Bark Beetles (Curculionidae: Scolytinae) and Associates in a Northern Arizona Ponderosa Pine Forest 1,2 1 3 1 M. L. GAYLORD, T. E. KOLB, K. F. WALLIN, AND M. R. WAGNER Environ. Entomol. 35(1): 37Ð47 (2006) ABSTRACT Ponderosa pine forests in northern Arizona have historically experienced limited bark beetle-caused tree mortality, and little is known about the bark beetle community in these forests. Our objectives were to describe the ßight seasonality and lure preference of bark beetles and their associates in these forests. We monitored bark beetle populations for 24 consecutive months in 2002 and 2003 using Lindgren funnel traps with Þve different pheromone lures. In both years, the majority of bark beetles were trapped between May and October, and the peak captures of coleopteran predator species, Enoclerus (F.) (Cleridae) and Temnochila chlorodia (Mannerheim), occurred between June and August. Trap catches of Elacatis (Coleoptera: Othniidae, now Salpingidae), a suspected predator, peaked early in the spring. For wood borers, trap catches of the Buprestidae family peaked in late May/early June, and catches of the Cerambycidae family peaked in July/August. The lure targeted for Dendroctonus brevicomis LeConte attracted the largest percentage of all Dendroc- tonus beetles except for D. valens LeConte, which was attracted in highest percentage to the lure targeted for D. valens. The lure targeted for Ips pini attracted the highest percentage of beetles for all three Ips species [I.pini (Say), I. latidens (LeConte), and I. lecontei Swaine] and the two predators, Enoclerus and T. chlorodia. -

Field Bioassays of Synthetic Pheromones and Host Monoterpenes for Conophthorus Coniperda (Coleoptera: Scolytidae)
PHYSIOLXICAL AND CHEMICAL ECOLOGY Field Bioassays of Synthetic Pheromones and Host Monoterpenes for Conophthorus coniperda (Coleoptera: Scolytidae) PETER DE GROOT,’ GART L. DEBARR,3 AND GORAN BIRGERSSON’,* Environ. Entomol. Z(2): 38-W (1998) ABSTRACT Four major monoterpenes, (i-)-cu-pinene, 1 (S)-( -)-/3-pinene, (R)-( t )-limonene, and myrcene are found in the cones of eastern white pines, Pinusstrobus L. Mixtures ofthese, as well as. u-pinene or P-pinene alone. increased catches of male white pine cone beetles, Conophthorus coniperda (Schwartz). in traps baited xvith the female sex pheromone, ( z)-trans-pityol. The mono- terpenes by themsekes as mistures or individually (a-pinene, /3-pinene) were not attractants for males or females. Traps baited with I r )-tram-pityol and wpinene caught as many, or significantly more beetles than those baited with pity01 and a four monoterpene mixture (1:l:l:l) used in seed orch‘ards in North Carolina, Ohio. and Virginia. Three beetle-produced compounds, conophthorin, tram-pinocarveol. and myrtenol did not enhance catches of males or females in ( z)-trans-pityol- baited traps. Racemic E-(k)- conophthorin. E-( -)- conophthorin, and E-( +)- conophthorin sig- nificantly reduced catches of males in traps baited with ( -c)-tran.s-pityol alone. Female C. coniperda were not attracted to any ofthe host- or beetle-produced compounds tested. The study demonstrated that traps \vith baits releasing (z)-t,ans-pityol at about lmgiwk with ( z)-a-pinene (98%pure) are potentially valuable tools for C conipwda pest management. Baited traps can be used to monitor C. cmipwdn populations or possibl>. to reduce seed losses in a beetle trap-out control strategy. -

Shade Tree Borers No
I N S E C T S E R I E S TREES & SHRUBS Shade Tree Borers no. 5.530 by W.S. Cranshaw and D.A. Leatherman 1 Shade tree borers are insects that develop underneath the bark of woody plants. Most of these insects can attack only dying trees, felled logs or trees under stress. Stress to woody plants may be the result of mechanical injury, recent transplanting, overwatering or drought. These borers often are incorrectly blamed Quick Facts... for damage caused by a pre-existing condition or injury. Certain borers, in particular the “clear-wing borers,” are capable of damaging apparently healthy trees. Shade tree borers are insects that develop underneath the Life History and Habits bark of trees and shrubs. Certain A large number of beetles and moths develop as wood borers in their beetles and moths are the most immature (larval) stage. When full-grown, typically in one to two years, the adult common borers. stages cut a hole through the bark and emerge. Many of the adult borers, particularly the longhorned beetles and Most shade tree borers can metallic wood borers, feed on pollen, tender bark or leaves but do not cause any successfully attack only trees significant injury. The adult stage is mostly spent flying to new host trees, mating that are injured or stressed. and laying eggs. Eggs of most shade tree borers are laid on the bark, usually within small Shade tree borer development cracks. Longhorned beetles and horntails deposit their eggs underneath bark. takes from one to three years to Eggs typically hatch within one to two weeks, and the newly emerged complete. -

Cone and Seed Insects of Southwestern White Pine Daniel E
Forest Insect & Disease Leaflet 189 March 2020 U.S. D ep ar t ment of Ag r ic u lture • Forest S er v ice Cone and Seed Insects of Southwestern White Pine Daniel E. DePinte1, Kristen M. Waring2, and Monica L. Gaylord3 Introduction Southwestern white pine, Pinus stro biformis Engelm. (SWWP), like other western pines, has a guild of insect spe cies that feed on its cones and seeds. Those described here are the most commonly observed pests with a his tory of causing damage to SWWP cone and seed production. They are taxo nomically diverse, and include species of Hemiptera, Diptera, Coleoptera, Lepidoptera, and Hymenoptera. Host Distribution Southwestern white pine is a five- needled pine found throughout Figure 1. Distribution of southwestern white mixed conifer forests of the American pine in U.S. and Mexico (Shirk et al 2018). Southwest and Sierra Madre Occidental It is a major source of sustenance Mountains of Mexico (Figure 1). In for wildlife with its relatively large, the United States SWWP typically nutrient rich seeds. SWWP has been co-occurs with other species; very known to hybridize with limber pine rarely occurring as a pure stand at (P. flexilis). SWWP is also susceptible elevations from 7,000 to 10,000 feet to the non-native invasive pathogen, above sea level. It plays a critical role Cronartium ribicola (J. C. Fisch), which in early seral stages of forest succes causes white pine blister rust. When a sion and is a vital component of mixed SWWP tree is approximately 15 years conifer forest types. -

Fungi Associated with the North American Spruce Beetle, Dendroctonus Rufipennis
Color profile: Generic CMYK printer profile Composite Default screen 1815 NOTE / NOTE Fungi associated with the North American spruce beetle, Dendroctonus rufipennis Diana L. Six and Barbara J. Bentz Abstract: Fungi were isolated from individual Dendroctonus rufipennis (Kirby) collected from six populations in Alaska, Colorado, Utah, and Minnesota, U.S.A. In all populations, Leptographium abietinum (Peck) Wingfield was the most commonly isolated mycelial fungus (91–100% of beetles). All beetles in all populations were associated with yeasts and some with only yeasts (0–5%). In one population, Ophiostoma ips (Rumbold) Nannf. was also present on 5% of the beetles but always in combination with L. abietinum and yeasts. Ophiostoma piceae (Munch) H. & P. Sydow was found on 2% of beetles in another population. Ceratocystis rufipenni Wingfield, Harrington & Solheim, previously reported as an associate of D. rufipennis, was not isolated from beetles in this study. Ceratocystis rufipenni is a viru- lent pathogen of host Picea, which has led to speculation that C. rufipenni aids the beetle in overcoming tree defenses and therefore contributes positively to the overall success of the beetle during colonization. However, our results, con- sidered along with those of others, indicate that C. rufipenni may be absent from many populations of D. rufipennis and may be relatively rare in those populations in which it is found. If this is true, C. rufipenni may be only a minor or incidental associate of D. rufipennis and, as such, not likely to have significant impacts on beetle success or popula- tion dynamics. Alternatively, the rarity of C. rufipenni in our and others isolations may be due to difficulties in isolat- ing this fungus in the presence of other faster growing fungi such as L. -
Common Names Working-2008Jan
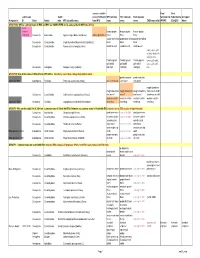
correct scientific Final Final submission Subfa name (if different WFI common ESC common ESA common Submitted to Submitted to Accepted Assigned to: ID Order Family mily WFI scientific name from WFI) name name name CABI name info? WFIWC: ESA/ESC: Name GROUP 000: Official common name in ESA and ESC are COMPLETED and is same as that in WFI (take off list) need change? bronze poplar bronze poplar bronze poplar 1026-Bup Coleoptera Buprestidae Agrilus liragus Barter and Brown Agrilus granulatus borer borer borer poplar-and-willow poplar-and-willow poplar-and-willow Coleoptera Curculionidae Cryptorhynchus [Sternochetus] lapathi (L.) borer borer borer Coleoptera Curculionidae Nemocestes incomptus (Horn) woods weevil woods weevil wood weevil cooley spruce gall adelgid; douglas fir adelges; sitka Cooley spruce Cooley spruce Cooley spruce spruce gall aphid; gall aphid gall aphid gall aphid spruce gall aphid, Homoptera Adelgidae Adelges cooleyi (Gillette) (adelgid) (adelgid) (adelgid) blue GROUP 00: One of the names in ESA, ESC or WFI differs (Decide by case; likely change highlighted name) ponderosa pine ponderosa pine approved 1989 Lepidoptera Pyralidae Dioryctria auranticella (Grote) pine coneworm coneworm coneworm rough strawberry rough strawberry rough strawberry rough strawberry root weevil; rough Coleoptera Curculionidae Otiorhynchus rugosostriatus (Goeze) root weevil weevil root weevil strawberry weevil western conifer western conifer- western conifer- western conifer- approved 1989 Hemiptera Coreidae Leptoglossus occidentalis Heidemann seed -

Bark Beetle (Curculionidae: Scolytinae) Record in the La Primavera Forest, Jalisco State
Revista Mexicana de Ciencias Forestales Vol. 9 (48) DOI: https://doi.org/10.29298/rmcf.v8i48.122 Article Bark beetle (Curculionidae: Scolytinae) record in the La Primavera Forest, Jalisco State Antonio Rodríguez-Rivas1* Sara Gabriela Díaz-Ramos1 Héctor Jesús Contreras-Quiñones1 Lucía Barrientos-Ramírez1 Teófilo Escoto García1 Armando Equihua-Martínez2 1Departamento de Madera, Celulosa y Papel, Centro Universitario de Ciencias Exactas e Ingenierías, Universidad de Guadalajara. México. 2Posgrado Fitosanidad, Colegio de Postgraduados, Campus Montecillos. México. *Autor por correspondencia; correo-e: [email protected] Abstract: The first registers of Scolytinae were obtained for the La Primavera Forest, Jalisco (a protected natural area), with 11 species and six genera, as well as their altitudinal distribution. The insects were captured by means of two Lindgren traps with ten funnels each (baited with Dendroctonus ponderosa and Ips typographus pheromones), installed on pine-oak vegetation, and three traps with the shape of a metal funnel (baited with 70 % ethyl alcohol and antifreeze on the outside, and thinner on the inside); of the latter, two were placed on pine and oak vegetation, and the third, in an acacia association. The five traps were distributed within an altitude range of 1 380 to 1 580 masl. The group that most abounded in bark beetle species included Xyleborus affinis, X. ferruginueus, X. volvulus and Gnathotrichus perniciosus. Three new species —Hylurgops subcostulatus alternans, Premnobius cavipenni and Xyleborus horridus— were collected and registered in the state of Jalisco, and two more —Ips calligraphus and I. cribicollis—, at a local level. The traps and baits elicited a good response and proved to be efficient for capturing bark beetle insects. -

Membership 2005: Year in Review
ESA Newsletter Information for the Members of the Entomological Society of America MARCH 2006 • VOLUME 29, NUMBER 3 Membership 2005: Year in Review By Chris Stelzig, Director of Membership we get too far into the year, I wanted to fin- not withstanding, this is a great, inexpensive and Marketing ish the update on 2005. way to get general feedback from you on a Sections and Branches—The Pacific wide range of topics. Headquarters uses this For the first time since 1992, ESA posted Branch saw the most growth when we com- data for planning purposes. In the Member- two back-to-back years of membership pare 2004 to 2005 with a 12% increase in ship Toolbox on the website (http://www. growth. This is a milestone, especially when membership. Only the Southeastern Branch entsoc.org/membership/toolbox/support_ coupled with the fact that just five years ago saw an actual decline, and that was merely esa/survey.htm), you will find a list of all our we were losing members by the hundreds by one person (a case in point to say that active surveys and an invitation to partici- every year and our funds were quickly dry- EVERY membership renewal is important!). pate in one. ing up. You’ve heard me say “Strength in Section B saw the largest growth last year, Performance—About 80% of members Numbers” for nearly five years now. The leaping more than 10%. who responded felt that they were satisfied reason for this is that membership is the Membership Types—As I mentioned with performance from ESA headquarters. -

Forest Insect Conditions in the United States 1966
FOREST INSECT CONDITIONS IN THE UNITED STATES 1966 FOREST SERVICE ' U.S. DEPARTMENT OF AGRICULTURE Foreword This report is the 18th annual account of the scope, severity, and trend of the more important forest insect infestations in the United States, and of the programs undertaken to check resulting damage and loss. It is compiled primarily for managers of public and private forest lands, but has become useful to students and others interested in outbreak trends and in the location and extent of pest populations. The report also makes possible n greater awareness of the insect prob lem and of losses to the timber resource. The opening section highlights the more important conditions Nationwide, and each section that pertains to a forest region is prefaced by its own brief summary. Under the Federal Forest Pest Control Act, a sharing by Federal and State Governments the costs of surveys and control is resulting in a stronger program of forest insect and disease detection and evaluation surveys on non-Federal lands. As more States avail themselves of this financial assistance from the Federal Government, damage and loss from forest insects will become less. The screening and testing of nonpersistent pesticides for use in suppressing forest defoliators continued in 1966. The carbamate insecticide Zectran in a pilot study of its effectiveness against the spruce budworm in Montana and Idaho appeared both successful and safe. More extensive 'tests are planned for 1967. Since only the smallest of the spray droplets reach the target, plans call for reducing the spray to a fine mist. The course of the fine spray, resulting from diffusion and atmospheric currents, will be tracked by lidar, a radar-laser combination. -

Pinus Contorta Dougl
Unclassified ENV/JM/MONO(2008)32 Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 05-Dec-2008 ___________________________________________________________________________________________ English - Or. English ENVIRONMENT DIRECTORATE JOINT MEETING OF THE CHEMICALS COMMITTEE AND Unclassified ENV/JM/MONO(2008)32 THE WORKING PARTY ON CHEMICALS, PESTICIDES AND BIOTECHNOLOGY Cancels & replaces the same document of 04 December 2008 Series on Harmonisation of Regulatory Oversight in Biotechnology No. 44 CONSENSUS DOCUMENT ON THE BIOLOGY OF LODGEPOLE PINE (Pinus contorta Dougl. ex. Loud.) English - Or. English JT03257048 Document complet disponible sur OLIS dans son format d'origine Complete document available on OLIS in its original format ENV/JM/MONO(2008)32 Also published in the Series on Harmonisation of Regulatory Oversight in Biotechnology: No. 1, Commercialisation of Agricultural Products Derived through Modern Biotechnology: Survey Results (1995) No. 2, Analysis of Information Elements Used in the Assessment of Certain Products of Modern Biotechnology (1995) No. 3, Report of the OECD Workshop on the Commercialisation of Agricultural Products Derived through Modern Biotechnology (1995) No. 4, Industrial Products of Modern Biotechnology Intended for Release to the Environment: The Proceedings of the Fribourg Workshop (1996) No. 5, Consensus Document on General Information concerning the Biosafety of Crop Plants Made Virus Resistant through Coat Protein Gene-Mediated Protection (1996) No. 6, Consensus Document on Information Used in the Assessment of Environmental Applications Involving Pseudomonas (1997) No. 7, Consensus Document on the Biology of Brassica napus L. (Oilseed Rape) (1997) No. 8, Consensus Document on the Biology of Solanum tuberosum subsp. tuberosum (Potato) (1997) No. 9, Consensus Document on the Biology of Triticum aestivum (Bread Wheat) (1999) No. -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture,