Forestry Research Notes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
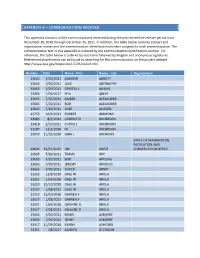
Appendix B – Communications Received
APPENDIX B – COMMUNICATIONS RECEIVED This appendix contains all the communications received during the post-centerline release period from November 18, 2010 through December 31, 2011. In addition, the table below contains contact and organization names and the communication identification number assigned to each communication. The communication text in this appendix is ordered by the communication identification number. For reference, the table below is ordered by last name followed by illegible and anonymous signatures. Referenced attachments can be found by searching for the communication on the project website http://www.bpa.gov/corporate/I-5-EIS/search.cfm. Number Date Name - First Name - Last Organization 13665 7/20/2011 ANDREW ABBOTT 13665 7/20/2011 JACK ABERNATHY 13665 7/20/2011 CRYSTAL L ADAMS 13395 1/29/2011 PHIL AKELY 13667 7/20/2011 AMBER ALEXANDER 13665 7/20/2011 BOB ALEXANDER 13665 7/20/2011 CHEE ALLISON 13755 10/6/2011 ROBERT AMMONS 13683 8/3/2011 CANDICE D ANDERSON 13418 2/10/2011 CURTIS L ANDERSON 13207 12/2/2010 M. ANDERSON 13073 11/22/2010 GINA L ANDREWS STATE OF WASHINGTON, RECREATION AND 13836 12/15/2011 JIM ANEST CONSERVATION OFFICE 13665 7/20/2011 TRAVIS APP 13665 7/20/2011 BOB APPLING 13665 7/20/2011 JEREMY ARIONUS 13665 7/20/2011 CHUCK ARNST 13322 12/8/2010 DALE W AROLA 13321 12/9/2010 DALE W AROLA 13320 12/10/2010 DALE W AROLA 13527 1/28/2011 DALE W AROLA 13320 12/10/2010 DARREN F AROLA 13527 1/28/2011 DARREN F AROLA 13321 12/9/2010 DWAYNE D AROLA 13527 1/28/2011 DWAYNE D AROLA 13665 7/20/2011 BRIAN ASBURRY 13665 -

Older Forests Used by Northern Spotted Owls Functioned As Re Refugia
Older forests used by northern spotted owls functioned as re refugia during large wildres, 1987–2017 Damon B Lesmeister ( [email protected] ) USDA Forest Service Pacic Northwest Research Station https://orcid.org/0000-0003-1102-0122 Raymond J. Davis USDA Forest Service Region 6: USDA Forest Service Pacic Northwest Region Stan G. Sovern USDA Forest Service Pacic Northwest Research Station Zhiqiang Yang USDA Forest Service Rocky Mountain Research Station Research Article Keywords: Northern spotted owl, Strix occidentalis caurina, wildre severity, RdNBR, climate change, re refugia Posted Date: March 12th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-280175/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/27 Abstract Background The northern spotted owl (Strix occidentalis caurina) is an Endangered Species Act-listed subspecies that requires forests with old-growth characteristics for nesting. With climate change, large, severe wildres are expected to be more common and an increasing threat to spotted owl persistence. Understanding re severity patterns related to nesting forest can be valuable for forest management that supports conservation and recovery, especially if nesting forest functions as re refugia (i.e., lower re severity than surrounding landscape). We examined the relationship between re severity and nesting forests in 472 large wildres (> 200 ha) that occurred rangewide during 1987–2017. We mapped re severities (unburned-low, moderate, high) within each re using relative difference normalized burn ratios and quantied differences in severity between pre-re nesting forest (edge and interior) and non-nesting forest. We also quantied these relationships within areas of three re regimes (low severity, very frequent; mixed severity, frequent; high severity, infrequent). -

RCFB April 2021 Page 1 Agenda TUESDAY, April 27 OPENING and MANAGEMENT REPORTS 9:00 A.M
REVISED 4/8/21 Proposed Agenda Recreation and Conservation Funding Board April 27, 2021 Online Meeting ATTENTION: Protecting the public, our partners, and our staff are of the utmost importance. Due to health concerns with the novel coronavirus this meeting will be held online. The public is encouraged to participate online and will be given opportunities to comment, as noted below. If you wish to participate online, please click the link below to register and follow the instructions in advance of the meeting. Technical support for the meeting will be provided by RCO’s board liaison who can be reached at [email protected]. Registration Link: https://zoom.us/webinar/register/WN_JqkQAGCrRSOwbHLmg3a6oA Phone Option: (669)900-6833 - Webinar ID: 967 5491 2108 Location: RCO will also have a public meeting location for members of the public to listen via phone as required by the Open Public Meeting Act, unless this requirement is waived by gubernatorial executive order. In order to enter the building, the public must not exhibit symptoms of the COVID-19 and will be required to comply with current state law around personal protective equipment. RCO staff will meet the public in front of the main entrance to the natural resources building and escort them in. *Additionally, RCO will record this meeting and would be happy to assist you after the meeting to gain access to the information. Order of Presentation: In general, each agenda item will include a short staff presentation and followed by board discussion. The board only makes decisions following the public comment portion of the agenda decision item. -

SWAP Full Document
Hudec et al. Front Matter 1 1 Climate Change Vulnerability and Adaptation in 2 Southwest Washington 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Editors 33 J.L. Hudec is an ecologist, U.S. Department of Agriculture, Forest Service, Gifford Pinchot 34 National Forest, Mt. Adams Ranger District, 2455 Hwy 141, Trout Lake, WA 98650; J.E. 35 Halofsky is a research ecologist, University of Washington, College of the Environment, School 36 of Environmental and Forest Sciences, Box 352100, Seattle, WA 98195-2100; D.L. Peterson is 37 a senior research biological scientist, U.S. Department of Agriculture, Forest Service, Pacific 38 Northwest Research Station, 400 N 34th St., Suite 201, Seattle, WA 98103; J.J. Ho is a research 39 economist, University of Washington, College of the Environment, School of Environmental and 40 Forest Sciences,DRAFT Box 352100, Seattle, WA 98195-2100. Hudec et al. Front Matter 2 41 Climate Change Vulnerability and Adaptation in 42 Southwest Washington 43 44 J.L. Hudec, J.E. Halofsky, D.L. Peterson, and J.J. Ho 45 46 47 Editors 48 49 50 51 52 53 U.S. Department of Agriculture, Forest Service 54 Pacific Northwest Research Station 55 Portland, Oregon 56 General Technical Report PNW-GTR-xxxx 57 Month year 58 DRAFT Hudec et al. Front Matter 3 59 Abstract 60 61 Hudec, J.L.; Halofsky, J.E.; Peterson, D.L.; Ho, J.J., eds. 201X. Climate change vulnerability 62 and adaptation in Southwest Washington. -

Population Dynamics of the Douglas-Fir Cone Moth, Barbara
AN ABSTRACT OF THE THESIS OF THOMAS EVAN NEBEKERfor the DOCTOR OF PHILOSOPHY (Name) (Degree) in ENTOMOLOGY presented on August 29, 1973 (Major) (Date) Title: POPULATION DYNAMICS OF THE DOUGLAS-FIR CONE MOTH BARBARA COLFAXIANA (KFT. ) (LEPIDOPTERA: OLETHR EUTIDAE) Redacted for Privacy Abstract approved; William P. Agel A population of the Douglas-fir cone moth, Barbara colfaxiana (Kft. ), was studied on the Buckhead Seed Production Area, Oakridge, Oregon, during 1971 and 1972.A method of estimating cone and insect populations is presented.Factors contributing to the mortality of B. colfaxiana are discussed, with resinosis being the critical factor. Larval food consumption is discussed with reference to calories of cone tissue consumed through time. An average of761 calories are consumed through the four larval stages. Amounts consumed of each structure (bracts, scales, seeds) are presented. A structural model is presented that depicts the role of B. colfaxiana in a natural stand. Population. Dynamics of the Douglas-fir Cone Moth, Barbara colfaxiana (Kft. ) (Lepidoptera: Olethreutidae) by Thomas Evan Nebeker A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Completed (August 29, 1973) Commencement June 1974 APPROVED: Redacted for Privacy Associate Professor of Entomology in charge of major Redacted for Privacy ---....- Department Chairman, Department of Entomology Redacted for Privacy Dean of Graduate School Date thesis is presented August 29, 1973 Typed byMildred N. Israelsenfor Thomas Evan Nebeker ACKNOWLEDGMENTS My endeavors have been enhanced by the advice, criticism, and encouragement of my committee and friends.I have been fortunate and happy in having Dr. -

Descripción De Nuevas Especies Animales De La Península Ibérica E Islas Baleares (1978-1994): Tendencias Taxonómicas Y Listado Sistemático
Graellsia, 53: 111-175 (1997) DESCRIPCIÓN DE NUEVAS ESPECIES ANIMALES DE LA PENÍNSULA IBÉRICA E ISLAS BALEARES (1978-1994): TENDENCIAS TAXONÓMICAS Y LISTADO SISTEMÁTICO M. Esteban (*) y B. Sanchiz (*) RESUMEN Durante el periodo 1978-1994 se han descrito cerca de 2.000 especies animales nue- vas para la ciencia en territorio ibérico-balear. Se presenta como apéndice un listado completo de las especies (1978-1993), ordenadas taxonómicamente, así como de sus referencias bibliográficas. Como tendencias generales en este proceso de inventario de la biodiversidad se aprecia un incremento moderado y sostenido en el número de taxones descritos, junto a una cada vez mayor contribución de los autores españoles. Es cada vez mayor el número de especies publicadas en revistas que aparecen en el Science Citation Index, así como el uso del idioma inglés. La mayoría de los phyla, clases u órdenes mues- tran gran variación en la cantidad de especies descritas cada año, dado el pequeño núme- ro absoluto de publicaciones. Los insectos son claramente el colectivo más estudiado, pero se aprecia una disminución en su importancia relativa, asociada al incremento de estudios en grupos poco conocidos como los nematodos. Palabras clave: Biodiversidad; Taxonomía; Península Ibérica; España; Portugal; Baleares. ABSTRACT Description of new animal species from the Iberian Peninsula and Balearic Islands (1978-1994): Taxonomic trends and systematic list During the period 1978-1994 about 2.000 new animal species have been described in the Iberian Peninsula and the Balearic Islands. A complete list of these new species for 1978-1993, taxonomically arranged, and their bibliographic references is given in an appendix. -

THIRTY-YEAR CLUB PGION Six U.S.Copst Svicgr
THIRTY-YEAR CLUB PGION Six U.S.cOPST SvIcGr VOL. XVII JUNE -1963 THE HEART OF THE TREE What does he plant who plant. * tree? He plants a friend of sun and sky; He plants the flag of breeea free; A shaft of beauty, towering high. He plants a home to heaven anigh, For song and mother-croon of bird In hushed and happy twilight heard. The treble of heaven's harmony - These things he plants who plants a tree What does he plant who plants a tree? He plants cool shade and tender rain, And seed and bud of days to be, And years that fade and flush again; He plants the glory of the plain; He plants the forest's heritage; The harvest of a coming age; The Joy that unborn eyes shall see - These things he plants who plants a tree. S What does he plant who plants a tree? He plants, in sap and leaf and wood, In love of home and loyalty And far-cast thoughts of civil good. His blessings on the neighborhood Who in the hollow of Hi. hand Holds all the growth of all our land. A nation', growth from sea to sea Stirs in hia heart who plante a tree. Henry- Cuyler Bunner S T I M B E R L I N E S June 1963 VOL. XVII - PUBLISHED ANNUALLY BY R-6 FOREST SERVICE 30-YEAR CLUB Editor - Frank Flack Consulting Editors Publication TH. Burgess Biographies - History....,..,.,.Kirk P. Cecil Obituaries .Leslie L. Colvill Photographs Victor H. Flach Policy J. -

RV Sites in the United States Location Map 110-Mile Park Map 35 Mile
RV sites in the United States This GPS POI file is available here: https://poidirectory.com/poifiles/united_states/accommodation/RV_MH-US.html Location Map 110-Mile Park Map 35 Mile Camp Map 370 Lakeside Park Map 5 Star RV Map 566 Piney Creek Horse Camp Map 7 Oaks RV Park Map 8th and Bridge RV Map A AAA RV Map A and A Mesa Verde RV Map A H Hogue Map A H Stephens Historic Park Map A J Jolly County Park Map A Mountain Top RV Map A-Bar-A RV/CG Map A. W. Jack Morgan County Par Map A.W. Marion State Park Map Abbeville RV Park Map Abbott Map Abbott Creek (Abbott Butte) Map Abilene State Park Map Abita Springs RV Resort (Oce Map Abram Rutt City Park Map Acadia National Parks Map Acadiana Park Map Ace RV Park Map Ackerman Map Ackley Creek Co Park Map Ackley Lake State Park Map Acorn East Map Acorn Valley Map Acorn West Map Ada Lake Map Adam County Fairgrounds Map Adams City CG Map Adams County Regional Park Map Adams Fork Map Page 1 Location Map Adams Grove Map Adelaide Map Adirondack Gateway Campgroun Map Admiralty RV and Resort Map Adolph Thomae Jr. County Par Map Adrian City CG Map Aerie Crag Map Aeroplane Mesa Map Afton Canyon Map Afton Landing Map Agate Beach Map Agnew Meadows Map Agricenter RV Park Map Agua Caliente County Park Map Agua Piedra Map Aguirre Spring Map Ahart Map Ahtanum State Forest Map Aiken State Park Map Aikens Creek West Map Ainsworth State Park Map Airplane Flat Map Airport Flat Map Airport Lake Park Map Airport Park Map Aitkin Co Campground Map Ajax Country Livin' I-49 RV Map Ajo Arena Map Ajo Community Golf Course Map -

Pseudotsuga Menziesii
SPECIAL PUBLICATION 4 SEPTEMBER 1982 INVERTEBRATES OF THE H.J. ANDREWS EXPERIMENTAL FOREST, WESTERN CASCADE MOUNTAINS, OREGON: A SURVEY OF ARTHROPODS ASSOCIATED WITH THE CANOPY OF OLD-GROWTH Pseudotsuga Menziesii D.J. Voegtlin FORUT REJEARCH LABORATORY SCHOOL OF FORESTRY OREGON STATE UNIVERSITY Since 1941, the Forest Research Laboratory--part of the School of Forestry at Oregon State University in Corvallis-- has been studying forests and why they are like they are. A staff or more than 50 scientists conducts research to provide information for wise public and private decisions on managing and using Oregons forest resources and operating its wood-using industries. Because of this research, Oregons forests now yield more in the way of wood products, water, forage, wildlife, and recreation. Wood products are harvested, processed, and used more efficiently. Employment, productivity, and profitability in industries dependent on forests also have been strengthened. And this research has helped Oregon to maintain a quality environment for its people. Much research is done in the Laboratorys facilities on the campus. But field experiments in forest genetics, young- growth management, forest hydrology, harvesting methods, and reforestation are conducted on 12,000 acres of School forests adjacent to the campus and on lands of public and private cooperating agencies throughout the Pacific Northwest. With these publications, the Forest Research Laboratory supplies the results of its research to forest land owners and managers, to manufacturers and users of forest products, to leaders of government and industry, and to the general public. The Author David J. Voegtlin is Assistant Taxonomist at the Illinois Natural History Survey, Champaign, Illinois. -

Lepidoptera: Tortricidae)
1 A molecular phylogeny of Cochylina, with confirmation of its relationship to Euliina 2 (Lepidoptera: Tortricidae) 3 4 John W. Brown*1, Leif Aarvik2, Maria Heikkilä3, Richard Brown4, and Marko Mutanen5 5 6 1 National Museum of Natural History, Smithsonian Institution, Washington, DC, USA, e-mail: 7 [email protected] 8 2 Natural History Museum, University of Oslo, Norway, e-mail: [email protected] 9 3 Finnish Museum of Natural History, LUOMUS, University of Helsinki, Helsinki, 00014, 10 Finland, e-mail: [email protected] 11 4 Mississippi Entomological Museum, Mississippi State, MS 39762, USA, e-mail: 12 [email protected] 13 5 Ecology and Genetics Research Unit, PO Box 3000, 90014, University of Oulu, Finland, e- 14 mail: [email protected] 15 *corresponding author 16 17 This is the peer reviewed version of the following article: Brown, J.W., Aarvik, L., Heikkilä, M., 18 Brown, R. and Mutanen, M. (2020), A molecular phylogeny of Cochylina, with confirmation of its 19 relationship to Euliina (Lepidoptera: Tortricidae). Syst Entomol, 45: 160-174., which has been 20 published in final form at https://doi.org/10.1111/syen.12385. 21 1 22 Abstract. We conducted a multiple-gene phylogenetic analysis of 70 species representing 24 23 genera of Cochylina and eight species representing eight genera of Euliina, and a maximum 24 likelihood analysis based on 293 barcodes representing over 220 species of Cochylina. The 25 results confirm the hypothesis that Cochylina is a monophyletic group embedded within a 26 paraphyletic Euliina. We recognize and define six major monophyletic lineages within 27 Cochylina: a Phtheochroa Group, a Henricus Group, an Aethes Group, a Saphenista Group, a 28 Phalonidia Group, and a Cochylis Group. -

The Insect Microcosm of Western Juniper Berries by Lindsay A
The Insect Microcosm of Western Juniper Berries By Lindsay A. Dimitri, Kirk C. Tonkel, William S. Longland, and Brian G. Rector On the Ground closure reducing availability of herbaceous understory plants • Expansion of western juniper has been a major to livestock and wildlife, and intense wildfires that result in concern of ranchers and managers working on conversion to invasive annual grasslands. Extensive efforts rangelands. have been made to remove western juniper and restore the • Insects and mites associated with juniper berries shrublands being replaced. Management practices such as can impact juniper seed production, but little is prescribed burning, mechanical removal (chaining, felling known about arthropods inhabiting western juni- with chainsaws) and herbicides are used to thin or eliminate per or their effects on seeds. juniper in a given area. Despite the extensive literature de- • Our study of insects and other arthropods found tailing western juniper expansion, there are many aspects of inside juniper berries at two sites in northeastern its ecology that remain understudied, including interactions California found 37 species of insects and one with seed predators and seed dispersers that are potentially mite species, ranging from those that eat berries important aspects of the ongoing expansion. Like other juni- or seeds to parasitoid insects that develop from per species, western juniper does not reproduce vegetatively eggs laid inside other insects, ultimately killing (for example, by root sprouting), so this expansion is exclu- their host, and hyperparasitoids that parasitize sively attributable to the establishment of new seedlings. other parasitoids. Therefore, documenting the seed and seedling ecology of • We identified several granivores that consume western juniper is essential to understanding the rapid ex- western juniper seeds and, when abundant, may pansion of this species. -

University of Alberta
University of Alberta Seasonal phenology and reproductive behaviour of Dioryctria species Zeller (Lepidoptera: Pyralidae) in British Columbian seed orchards by Caroline Marie Whitehouse A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Master of Science in Ecology Department of Biological Sciences ©Caroline M. Whitehouse Spring 2011 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Library and Archives Bibliothèque et Canada Archives Canada Published Heritage Direction du Branch Patrimoine de l’édition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre référence ISBN: 978-0-494-70885-9 Our file Notre référence ISBN: 978-0-494-70885-9 NOTICE: AVIS: The author has granted a non- L’auteur a accordé une licence non exclusive exclusive license allowing Library and permettant