First Record of Sphecodina Caudata (Bremer & Grey, 1852)(Lepidoptera, Sphingidae) from Amur Oblast, with an Overview of Its Distribution in Russia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Hymenoptera, Symphyta) Большехехцирского Заповедника С
Амурский зоологический журнал, 2019, том XI, № 1 Amurian Zoological Journal, 2019, vol. XI, no. 1 www.azjournal.ru УДК 595.793 DOI: 10.33910/1999-4079-2019-11-1-72-77 http://www.zoobank.org/References/3BCBB470-E17E-4207-9DA4-F6529D369A52 ДОПОЛНЕНИЯ И ИСПРАВЛЕНИЯ К СПИСКУ ПИЛИЛЬЩИКОВ (HYMENOPTERA, SYMPHYTA) БОЛЬШЕХЕХЦИРСКОГО ЗАПОВЕДНИКА С. В. Василенко Институт систематики и экологии животных Сибирского отделения РАН, ул. Фрунзе, д. 11, Новосибирск, 630091, Россия Сведения об авторе Аннотация. Приводится 16 видов пилильщиков. Среди них 13 видов ранее Василенко Сергей Владимирович не отмечались на территории Большехехцирского заповедника. Acantholyda E-mail: [email protected] parki Shinohara et Byun, 1996, Arge disparilis (Kirby, 1882), A. flavomixta (André, SPIN-код: 9176-8171 1881), A. nigrovaginata Malaise, 1931, Strongylogaster empriaeformis (Malaise, 1931), Macrophya sanguinolenta (Gmelin, 1790), M. vacillans Malaise, 1931, Rhogogaster chlorosoma (Benson, 1943) и R. sibirica Enslin, 1912 впервые обнаружены в Хабаровском крае. A. hasegawae Takeuchi, 1927 впервые найден в Хабаровском крае и на о-ве Кунашир. Обсуждаются некоторые проблемы, связанные с сибирскими и дальневосточными видами рода Rhogogaster Konow, 1884. Ключевые слова: пилильщики, новые находки, Большехехцирский заповедник, Дальний Восток. AMENDMENTS TO THE LIST OF SAWFLIES (HYMENOPTERA, SYMPHYTA) OF THE BOLSHEKHEKHTSIRSKII RESERVE S. V. Vasilenko Institute of Animal Systematics and Ecology, Siberian Branch of the Russian Academy of Sciences, 11 Frunze Str., Novosibirsk, 630091, Russia Author Abstract. The paper lists 16 species of sawflies of which 13 species were Sergey V. Vasilenko discovered on the territory of the Bolshekhekhtsirskii reserve for the first E-mail: [email protected] time. Acantholyda parki Shinohara et Byun, 1996, Arge disparilis (Kirby, SPIN: 9176-8171 1882), A. -

Butterflies of Ontario & Summaries of Lepidoptera
ISBN #: 0-921631-12-X BUTTERFLIES OF ONTARIO & SUMMARIES OF LEPIDOPTERA ENCOUNTERED IN ONTARIO IN 1991 BY A.J. HANKS &Q.F. HESS PRODUCTION BY ALAN J. HANKS APRIL 1992 CONTENTS 1. INTRODUCTION PAGE 1 2. WEATHER DURING THE 1991 SEASON 6 3. CORRECTIONS TO PREVIOUS T.E.A. SUMMARIES 7 4. SPECIAL NOTES ON ONTARIO LEPIDOPTERA 8 4.1 The Inornate Ringlet in Middlesex & Lambton Cos. 8 4.2 The Monarch in Ontario 8 4.3 The Status of the Karner Blue & Frosted Elfin in Ontario in 1991 11 4.4 The West Virginia White in Ontario in 1991 11 4.5 Butterfly & Moth Records for Kettle Point 11 4.6 Butterflies in the Hamilton Study Area 12 4.7 Notes & Observations on the Early Hairstreak 15 4.8 A Big Day for Migrants 16 4.9 The Ocola Skipper - New to Ontario & Canada .17 4.10 The Brazilian Skipper - New to Ontario & Canada 19 4.11 Further Notes on the Zarucco Dusky Wing in Ontario 21 4.12 A Range Extension for the Large Marblewing 22 4.13 The Grayling North of Lake Superior 22 4.14 Description of an Aberrant Crescent 23 4.15 A New Foodplant for the Old World Swallowtail 24 4.16 An Owl Moth at Point Pelee 25 4.17 Butterfly Sampling in Algoma District 26 4.18 Record Early Butterfly Dates in 1991 26 4.19 Rearing Notes from Northumberland County 28 5. GENERAL SUMMARY 29 6. 1990 SUMMARY OF ONTARIO BUTTERFLIES, SKIPPERS & MOTHS 32 Hesperiidae 32 Papilionidae 42 Pieridae 44 Lycaenidae 48 Libytheidae 56 Nymphalidae 56 Apaturidae 66 Satyr1dae 66 Danaidae 70 MOTHS 72 CONTINUOUS MOTH CYCLICAL SUMMARY 85 7. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -
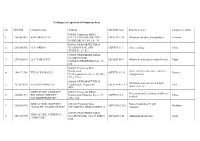
Catalogue of Exporters of Primorsky Krai № ITN/TIN Company Name Address OKVED Code Kind of Activity Country of Export 1 254308
Catalogue of exporters of Primorsky krai № ITN/TIN Company name Address OKVED Code Kind of activity Country of export 690002, Primorsky KRAI, 1 2543082433 KOR GROUP LLC CITY VLADIVOSTOK, PR-T OKVED:51.38 Wholesale of other food products Vietnam OSTRYAKOVA 5G, OF. 94 690001, PRIMORSKY KRAI, 2 2536266550 LLC "SEIKO" VLADIVOSTOK, STR. OKVED:51.7 Other ratailing China TUNGUS, 17, K.1 690003, PRIMORSKY KRAI, VLADIVOSTOK, 3 2531010610 LLC "FORTUNA" OKVED: 46.9 Wholesale trade in specialized stores China STREET UPPERPORTOVA, 38- 101 690003, Primorsky Krai, Vladivostok, Other activities auxiliary related to 4 2540172745 TEK ALVADIS LLC OKVED: 52.29 Panama Verkhneportovaya street, 38, office transportation 301 p-303 p 690088, PRIMORSKY KRAI, Wholesale trade of cars and light 5 2537074970 AVTOTRADING LLC Vladivostok, Zhigura, 46 OKVED: 45.11.1 USA motor vehicles 9KV JOINT-STOCK COMPANY 690091, Primorsky KRAI, Processing and preserving of fish and 6 2504001293 HOLDING COMPANY " Vladivostok, Pologaya Street, 53, OKVED:15.2 China seafood DALMOREPRODUKT " office 308 JOINT-STOCK COMPANY 692760, Primorsky Krai, Non-scheduled air freight 7 2502018358 OKVED:62.20.2 Moldova "AVIALIFT VLADIVOSTOK" CITYARTEM, MKR-N ORBIT, 4 transport 690039, PRIMORSKY KRAI JOINT-STOCK COMPANY 8 2543127290 VLADIVOSTOK, 16A-19 KIROV OKVED:27.42 Aluminum production Japan "ANKUVER" STR. 692760, EDGE OF PRIMORSKY Activities of catering establishments KRAI, for other types of catering JOINT-STOCK COMPANY CITYARTEM, STR. VLADIMIR 9 2502040579 "AEROMAR-ДВ" SAIBEL, 41 OKVED:56.29 China Production of bread and pastry, cakes 690014, Primorsky Krai, and pastries short-term storage JOINT-STOCK COMPANY VLADIVOSTOK, STR. PEOPLE 10 2504001550 "VLADHLEB" AVENUE 29 OKVED:10.71 China JOINT-STOCK COMPANY " MINING- METALLURGICAL 692446, PRIMORSKY KRAI COMPLEX DALNEGORSK AVENUE 50 Mining and processing of lead-zinc 11 2505008358 " DALPOLIMETALL " SUMMER OCTOBER 93 OKVED:07.29.5 ore Republic of Korea 692183, PRIMORSKY KRAI KRAI, KRASNOARMEYSKIY DISTRICT, JOINT-STOCK COMPANY " P. -

Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2
Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) by Boris C. Kondratieff, Paul A. Opler, Matthew C. Garhart, and Jason P. Schmidt C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 March 15, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration (top to bottom): Widow Skimmer (Libellula luctuosa) [photo ©Robert Behrstock], Stonefly (Perlesta species) [photo © David H. Funk, White- lined Sphinx (Hyles lineata) [photo © Matthew C. Garhart] ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences, Colorado State University, Fort Collins, Colorado 80523 Copyrighted 2004 Table of Contents EXECUTIVE SUMMARY……………………………………………………………………………….…1 INTRODUCTION…………………………………………..…………………………………………….…3 OBJECTIVE………………………………………………………………………………………….………5 Site Descriptions………………………………………….. METHODS AND MATERIALS…………………………………………………………………………….5 RESULTS AND DISCUSSION………………………………………………………………………..…...11 Dragonflies………………………………………………………………………………….……..11 -

Subterranean Fauna from Siberia and Russian Far East ______ENCYCLOPAEDIA BIOSPEOLOGICA (Siberia-Far East Special Issue)
Research Article ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.biotaxa.org/em Subterranean fauna from Siberia and Russian Far East _________________ ENCYCLOPAEDIA BIOSPEOLOGICA (Siberia-Far East special Issue) CHRISTIAN JUBERTHIE1, DIMITRI SIDOROV2, VASILE DECU3, ELENA MIKHALJOVA2 & KSENIA SEMENCHENKO2 1Encyclopédie Biospéologique, Edition. 1 Impasse Saint-Jacques, 09190 Saint-Lizier France; e-mail: [email protected] 2Institute of Biology and Soil Science, 100-letiya Vladisvostoka Av. 159, 690022 Vladivostok, Russia; e-mail: [email protected] 3Institutul de Speologie "Emil Racovitza", Academia romana, Calea 13 Septembrie, 13050711 Bucarest, Roumanie Received 20 March 2016 │ Accepted 25 November 2016 │ Published online 29 November 2016. Abstract Description of the main karstic regions of Siberia and Far East, and the most important caves. Survey of the subterranean species collected in caves, springs, hyporheic and MSSh. Relationship with the climate and glacial paleoclimatic periods to explain the paucity of the terrestrial fauna of Siberia. Persistence of some aquatic stygobionts (Crustacea), and richness of the subterranean fauna of the Far East, particularily in the Sikhote-Alin. The Crutaceans of the eastern part of the Ussury basin and Sakhalin Island have relationship with the Japanese and Korean fauna. Key words: karst, caves, springs, MSS, subterranean fauna, biogeography. 1 Generalities and History The study of caves in Siberia was begun in the late 17th century (Tsykin et al., 1979), but the first published report were made as early as in the 18th century by swedish geographer P. von Strahlenberg who in 1722 visited the cave on the Yenisei river bank above Krasnoyarsk and gave a short description, which is considered the first report of caves in Siberia (Strahlenberg, 1730). -

Russia) Biodiversity
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at SCHLOTGAUER • Anthropogenic changes of Priamurje biodiversity STAPFIA 95 (2011): 28–32 Anthropogenic Changes of Priamurje (Russia) Biodiversity S.D. SCHLOTGAUER* Abstract: The retrospective analysis is focused on anthropogenic factors, which have formed modern biodiversity and caused crucial ecological problems in Priamurje. Zusammenfassung: Eine retrospektive Analyse anthropogener Faktoren auf die Biodiversität und die ökologischen Probleme der Region Priamurje (Russland) wird vorgestellt . Key words: Priamurje, ecological functions of forests, ecosystem degradation, forest resource use, bioindicators, rare species, agro-landscapes. * Correspondence to: [email protected] Introduction Our research was focused on revealing current conditions of the vegetation cover affected by fires and timber felling. Compared to other Russian Far Eastern territories the Amur Basin occupies not only the vastest area but also has a unique geographical position as being a contact zone of the Circum- Methods boreal and East-Asian areas, the two largest botanical-geograph- ical areas on our planet. Such contact zones usually contain pe- The field research was undertaken in three natural-historical ripheral areals of many plants as a complex mosaic of ecological fratries: coniferous-broad-leaved forests, spruce and fir forests conditions allows floristic complexes of different origin to find and larch forests. The monitoring was carried out at permanent a suitable habitat. and temporary sites in the Amur valley, in the valleys of the The analysis of plant biodiversity dynamics seems necessary Amur biggest tributaries (the Amgun, Anui, Khor, Bikin, Bira, as the state of biodiversity determines regional population health Bureyza rivers) and in such divines as the Sikhote-Alin, Myao and welfare. -

Information for Persons Who Wish to Seek Asylum in the Russian Federation
INFORMATION FOR PERSONS WHO WISH TO SEEK ASYLUM IN THE RUSSIAN FEDERATION “Everyone has the right to seek and to enjoy in the other countries asylum from persecution”. Article 14 Universal Declaration of Human Rights I. Who is a refugee? According to Article 1 of the Federal Law “On Refugees”, a refugee is: “a person who, owing to well‑founded fear of being persecuted for reasons of race, religion, nationality, membership of particular social group or politi‑ cal opinion, is outside the country of his nationality and is unable or, owing to such fear, is unwilling to avail himself of the protection of that country”. If you consider yourself a refugee, you should apply for Refugee Status in the Russian Federation and obtain protection from the state. If you consider that you may not meet the refugee definition or you have already been rejected for refugee status, but, nevertheless you can not re‑ turn to your country of origin for humanitarian reasons, you have the right to submit an application for Temporary Asylum status, in accordance to the Article 12 of the Federal Law “On refugees”. Humanitarian reasons may con‑ stitute the following: being subjected to tortures, arbitrary deprivation of life and freedom, and access to emergency medical assistance in case of danger‑ ous disease / illness. II. Who is responsible for determining Refugee status? The responsibility for determining refugee status and providing le‑ gal protection as well as protection against forced return to the country of origin lies with the host state. Refugee status determination in the Russian Federation is conducted by the Federal Migration Service (FMS of Russia) through its territorial branches. -

° 2013 Annual Report ° Conservation Projects in the Russian Far East
° 2013 AnnuAl report ° ConservAtion projeCts in the russiAn FAr eAst Office 409, 2 Petra Velikogo Street Vladivostok, Russia 690091 Tel: +7 (423) 220-50-53 Fax: +7 (423) 220-50-48 E-mail: [email protected] Web-site: www.fundphoenix.org Annual report 2013 [PHOENIX FUND] Annual report 2013 BACKGrounD South of the Russian Far East represents the only area in the world where the Amur tigers and leopards still exist in the wild. The species are listed as Endangered by the IUCN and are on CITES Appendix I for protection status. Protected under the Russian and international laws and regulations, these rare predator populations are still threatened by poaching, habitat destruction, prey depletion and conflicts with people. For sixteen years the Phoenix Fund, Russian environmental NGO, has been conducting anti-poaching and habitat protection, environmental education and outreach, monitoring of industrial projects, paying compensations for livestock depredation in order to keep stable the Amur tiger and leopard populations. Below, we are glad to present our final report describing the project activities between January 1 and December 30, 2013. The activities described below are the result of joint efforts of many organizations, both Russian and international, and invaluable contribution of our supporters! [PHOENIX FUND] Annual report 2013 news in tiGer poliCy The year 2013 was declared by Vladimir Putin behind bars. On July 2, 2013, a new article 258.1 as the Year of Environment Protection in Russia. was introduced in the Russian Criminal Code We are glad to witness the ongoing steps by that envisages criminal responsibility for Russian Government to protect Amur tigers and poaching, keeping, acquisition, storage, leopards from extinction. -

The Mcguire Center for Lepidoptera and Biodiversity
Supplemental Information All specimens used within this study are housed in: the McGuire Center for Lepidoptera and Biodiversity (MGCL) at the Florida Museum of Natural History, Gainesville, USA (FLMNH); the University of Maryland, College Park, USA (UMD); the Muséum national d’Histoire naturelle in Paris, France (MNHN); and the Australian National Insect Collection in Canberra, Australia (ANIC). Methods DNA extraction protocol of dried museum specimens (detailed instructions) Prior to tissue sampling, dried (pinned or papered) specimens were assigned MGCL barcodes, photographed, and their labels digitized. Abdomens were then removed using sterile forceps, cleaned with 100% ethanol between each sample, and the remaining specimens were returned to their respective trays within the MGCL collections. Abdomens were placed in 1.5 mL microcentrifuge tubes with the apex of the abdomen in the conical end of the tube. For larger abdomens, 5 mL microcentrifuge tubes or larger were utilized. A solution of proteinase K (Qiagen Cat #19133) and genomic lysis buffer (OmniPrep Genomic DNA Extraction Kit) in a 1:50 ratio was added to each abdomen containing tube, sufficient to cover the abdomen (typically either 300 µL or 500 µL) - similar to the concept used in Hundsdoerfer & Kitching (1). Ratios of 1:10 and 1:25 were utilized for low quality or rare specimens. Low quality specimens were defined as having little visible tissue inside of the abdomen, mold/fungi growth, or smell of bacterial decay. Samples were incubated overnight (12-18 hours) in a dry air oven at 56°C. Importantly, we also adjusted the ratio depending on the tissue type, i.e., increasing the ratio for particularly large or egg-containing abdomens. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Lepidoptera für 1903. Bearbeitet von Dr. Robert Lucas in Rixdorf bei Berlin. A. Publikationen (Autoren alphabetisch) mit Referaten. Adkin, Robert. Pyrameis cardui, Plusia gamma and Nemophila noc- tuella. The Entomologist, vol. 36. p. 274—276. Agassiz, G. Etüde sur la coloration des ailes des papillons. Lausanne, H. Vallotton u. Toso. 8 °. 31 p. von Aigner-Abafi, A. (1). Variabilität zweier Lepidopterenarten. Verhandlgn. zool.-bot. Ges. Wien, 53. Bd. p. 162—165. I. Argynnis Paphia L. ; IL Larentia bilineata L. — (2). Protoparce convolvuli. Entom. Zeitschr. Guben. 17. Jahrg. p. 22. — (3). Über Mimikry. Gaea. 39. Jhg. p. 166—170, 233—237. — (4). A mimicryröl. Rov. Lapok, vol. X, p. 28—34, 45—53 — (5). A Mimicry. Allat. Kozl. 1902, p. 117—126. — (6). (Über Mimikry). Allgem. Zeitschr. f. Entom. 7. Bd. (Schluß p. 405—409). Über Falterarten, welche auch gesondert von ihrer Umgebung, in ruhendem Zustande eine eigentümliche, das Auge täuschende Form annehmen (Lasiocampa quercifolia [dürres Blatt], Phalera bucephala [zerbrochenes Ästchen], Calocampa exoleta [Stück morschen Holzes]. — [Stabheuschrecke, Acanthoderus]. Raupen, die Meister der Mimikry sind. Nachahmung anderer Tiere. Die Mimik ist in vielen Fällen zwecklos. — Die wenn auch recht geistreichen Mimikry-Theorien sind doch vielleicht nur ein müßiges Spiel der Phantasie. Aitken u. Comber, E. A list of the butterflies of the Konkau. Journ. Bombay Soc. vol. XV. p. 42—55, Suppl. p. 356. Albisson, J. Notes biologiques pour servir ä l'histoire naturelle du Charaxes jasius. Bull. Soc. Etud. Sc. nat. Nimes. T. 30. p. 77—82. Annandale u. Robinson. Siehe unter S w i n h o e. -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10