Frontiers in Life Science (Volume III) (ISBN: 978-81-953600-3-1)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diptera, Cecidomyiidae, Oligotrophini) with Description of G
Entomologica, XXII, Bari , 20-Xll-1987 E. SYLVÉW -M. SOLINAS 2 Structural and systematic review of Gephyraulus Riibsaamen (Diptera, Cecidomyiidae, Oligotrophini) with description of G. moricandiae sp. n. from Tunisia ABSTRA CT - The genus Gephyraulus Riibsaamen, 1915, is h ere redescribed. In addition to the type species , G. raphanistri (Kieffer), two species have been assigned to this genus : G. diplotaxis (Solinas), here transferred from Paragephyrau/us Solinas, 1982 , and G. moricandiae sp . n. In particular, the genus is characterized by the shape of the female uromeres VII and VIII, rogether forming (in resting position) a conspicuous swelling comaining most of the muscles for the regulation of the movemems of the oviposiror. The three species consti tute a distinct monophyletic group as indicated by obvious synapomorphies. Ali these species display a common behaviour as flower bud gall-makers on cruciferous plants. Key words: Cecidomyiidae, Gephyrau/us, functional anaromy, taxonomy, phylogeny. CONTENTS l. Imroduction 2. Methods, specimens examined, explanation of symbols 3. Results and discussion 3.1 Description survey 3.1.1 Redescription of Gephyraulus Riibsaamen , 1915 3.1.2 Description of Gephyraulus moricandiae sp. n. 3.1.3 Distinguishing characters on species leve! 3.2 Host plants and geographical disrribution 3.3 Preoviposiror functional unir 3.4 Phylogenetic aspects 4. Acknowledgements l. INTRODUCTION R OBSAAMEN (1915) established the genus Gephyraulus indicating as a peculiar feature: «die obere Lamelle der Legerohre cles 9 kurz; das letzte Glied oberseits mit einer Chitinspange, die sich bis iiber die Mitte der Lamelle hinzieht». He 1 Swedish Museum ofNaturai History, Department ofEntomology , S-1 0405 Srockholm, Sweden. -

Phylogeny and Host Association in Platygaster Latreille, 1809 (Hymenoptera, Platygastridae)
Phylogeny and host association in Platygaster Latreille, 1809 (Hymenoptera, Platygastridae) Peter Neerup Buhl Buhl, P.N.: Phylogeny and host association in Platygaster Latreille, 1809 (Hy menoptera, Platygastridae). Ent. Meddr 69: 113-122. Copenhagen, Denmark 2001. ISSN 0013-8851. An examination of the known midge host/midge plant host associations for species of Platygasterparasitoid wasps seems to indicate a number of natural par asitoid species groups restricted to specific plant families. Midge hosts seem less indicative for platygastrid relationships, but several exceptions from this rule exist. The possible reasons for this are discussed. It is also shown that species of Platy gaster with known host associations generally prefer midges on plant families which are not the families generally prefered by the midges. Furthermore, a com parison of the known midge host/midge plant host associations for the genera of the "Platygaster-cluster" and the "Synopeas-cluster" shows great differences in the general preferences of the clusters. P.N. Buhl, Troldh0jvej 3, DK-3310 0lsted, Denmark. E-mail: [email protected] Introduction The phylogeny of the very large platygastrid genus Platygaster, tiny parasitoids on gall midges (Diptera, Cecidomyiidae), is mostly unresolved. The great problems which meet the investigator are primarily - as in all platygastrids - the few external characters avail able in a phylogenetic analysis. A further obstacle in the revisionary work is that many species are known only from short dated original descriptions (unknown or unrevised type material). Aspects of the biology (midge host or host plant of midge) are, however, known for about half the described species, so perhaps this could enlighten aspects of the parasitoid taxonomy- as was successfully done e.g. -

Klicken, Um Den Anhang Zu Öffnen
Gredleria- VOL. 1 / 2001 Titelbild 2001 Posthornschnecke (Planorbarius corneus L.) / Zeichnung: Alma Horne Volume 1 Impressum Volume Direktion und Redaktion / Direzione e redazione 1 © Copyright 2001 by Naturmuseum Südtirol Museo Scienze Naturali Alto Adige Museum Natöra Südtirol Bindergasse/Via Bottai 1 – I-39100 Bozen/Bolzano (Italien/Italia) Tel. +39/0471/412960 – Fax 0471/412979 homepage: www.naturmuseum.it e-mail: [email protected] Redaktionskomitee / Comitato di Redazione Dr. Klaus Hellrigl (Brixen/Bressanone), Dr. Peter Ortner (Bozen/Bolzano), Dr. Gerhard Tarmann (Innsbruck), Dr. Leo Unterholzner (Lana, BZ), Dr. Vito Zingerle (Bozen/Bolzano) Schriftleiter und Koordinator / Redattore e coordinatore Dr. Klaus Hellrigl (Brixen/Bressanone) Verantwortlicher Leiter / Direttore responsabile Dr. Vito Zingerle (Bozen/Bolzano) Graphik / grafica Dr. Peter Schreiner (München) Zitiertitel Gredleriana, Veröff. Nat. Mus. Südtirol (Acta biol. ), 1 (2001): ISSN 1593 -5205 Issued 15.12.2001 Druck / stampa Gredleriana Fotolito Varesco – Auer / Ora (BZ) Gredleriana 2001 l 2001 tirol Die Veröffentlichungsreihe »Gredleriana« des Naturmuseum Südtirol (Bozen) ist ein Forum für naturwissenschaftliche Forschung in und über Südtirol. Geplant ist die Volume Herausgabe von zwei Wissenschaftsreihen: A) Biologische Reihe (Acta Biologica) mit den Bereichen Zoologie, Botanik und Ökologie und B) Erdwissenschaftliche Reihe (Acta Geo lo gica) mit Geologie, Mineralogie und Paläontologie. Diese Reihen können jährlich ge mein sam oder in alternierender Folge erscheinen, je nach Ver- fügbarkeit entsprechender Beiträge. Als Publikationssprache der einzelnen Beiträge ist Deutsch oder Italienisch vorge- 1 Naturmuseum Südtiro sehen und allenfalls auch Englisch. Die einzelnen Originalartikel erscheinen jeweils Museum Natöra Süd Museum Natöra in der eingereichten Sprache der Autoren und sollen mit kurzen Zusammenfassun- gen in Englisch, Italienisch und Deutsch ausgestattet sein. -
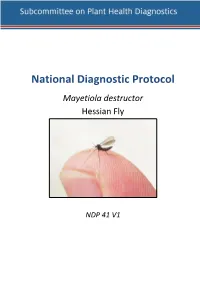
NDP 41 Hessian
NDP 41 V1- National Diagnostic Protocol for Mayetiola destructor National Diagnostic Protocol Mayetiola destructor Hessian Fly NDP 41 V1 NDP 41 V1 - National Diagnostic Protocol for Mayetiola destructor © Commonwealth of Australia Ownership of intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth). Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from http://creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from https://creativecommons.org/licenses/by/3.0/au/legalcode. This publication (and any material sourced from it) should be attributed as: Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee on Plant Health Diagnostics) Authors Severtson, D, Szito, A.; Reviewers Nicholas, A, Kehoe, M. ISBN 978-0-6481143-3-8 CC BY 3.0. Cataloguing data Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee -

Taxonomy of Janetiella Thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA Systematic Entomology Laboratory Entomology Collections, Miscellaneous 2009 Taxonomy of Janetiella thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae) Raymond Gagne Systematic Entomology Laboratory, PSI, Agricultural Research Service, USDA, c/o U. S. National Museum NHB 168, P.O. Box 37012, Washington, DC 20013-7012, USA, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/systentomologyusda Part of the Entomology Commons Gagne, Raymond, "Taxonomy of Janetiella thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae)" (2009). USDA Systematic Entomology Laboratory. 21. https://digitalcommons.unl.edu/systentomologyusda/21 This Article is brought to you for free and open access by the Entomology Collections, Miscellaneous at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA Systematic Entomology Laboratory by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PROC. ENTOMOL. SOC. WASH. 111(2), 2009, pp. 399–409 TAXONOMY OF JANETIELLA THYMI (KIEFFER) (DIPTERA: CECIDOMYIIDAE) AND OF THE SPECIES FORMERLY IN JANETIELLA THAT FEED ON VITIS (VITACEAE) RAYMOND J. GAGNE´ Systematic Entomology Laboratory, PSI, Agricultural Research Service, U.S. Department of Agriculture, c/o Smithsonian Institution MRC-168, P.O. Box 37012, Washington, DC 20013-7012, U.S.A. (e-mail: [email protected]) Abstract.—The poorly known European species Janetiella thymi (Kieffer), type species of Janetiella Kieffer (Diptera: Cecidomyiidae), is redescribed. Gall makers on grape that were formerly placed in Janetiella are shown to be distinct from that genus and transferred to Vitisiella Fedotova & Kovalev, a genus recently erected for a species on grape in Siberia. -

Spurgia Esulae: Permit Application Information Supplement
Reprinted from:. Release of nonindigenous biological control agents: Permit application Spurgia esulae: Permit application information supplement R. W. HANSEN USDA/APHIS/PPQ, Bozeman, MT (Article begins on the following page.) Page 1 of 15 Release of nonindigenous biological control agents: Permit application information supplement USDA-APHIS-PPQ-BATS Spurgia esulae (Diptera: Cecidomyiidae) 1. Proposed action Field release of Spurgia esulae Gagné (Diptera: Cecidomyiidae) for the biological control of the exotic weed, leafy spurge (Euphorbia esula L.), in the United States 2. Details of proposed action 2.1 Purpose of the release(s) Releases of Spurgia esulae will be used to initiate or augment populations at field insectary sites (FIS) in various states. Once these FIS populations are successfully established and are deemed sufficiently large, Spurgia esulae will be collected and distributed to leafy spurge-infested areas throughout the state. Spurgia esulae attacks the meristematic tissues (buds) of leafy spurge shoots, causing the formation of a gall (Gagné 1990). This damage rarely kills spurge shoots, but typically prevents flowering and, hence, seed production. Galls may also serve to divert nutrients from other plant tissues (Weis et al. 1988). Thus, the primary role of Spurgia esulae in the leafy spurge biocontrol program is to reduce seed production in spurge stands. A secondary role for this agent may lie in exerting a physiological stress on leafy spurge plants that could enhance the efficacy of other biological control agents. 2.2 Need for release Leafy spurge is a perennial herbaceous plant native to Europe and Asia. Since its accidental introduction beginning in the nineteenth century (Dunn 1985), leafy spurge has become a widespread and economically-important weed in the northern United States and in Canada. -

Nine New Species of Dasineura (Diptera: Cecidomyiidae) from Flowers of Australian Acacia (Mimosaceae)
Systematic Entomology (2005), DOI: 10.1111/j.1365-3113.2005.00287.x Nine new species of Dasineura (Diptera: Cecidomyiidae) from flowers of Australian Acacia (Mimosaceae) PETER KOLESIK1 , ROBIN J. ADAIR2 * andGEETA EICK3 1School of Earth and Environmental Sciences, Discipline of Soil and Land Systems, University of Adelaide, Adelaide, Australia, 2Plant Protection Research Institute, Agricultural Research Council, Stellenbosch, South Africa 3Department of Zoology, Evolutionary Genomics Group, University of Stellenbosch, Maitland, South Africa Abstract. Thirteen species of Australian acacias are invasive plants in agricultural and native vegetation areas of South Africa. Biological control programmes for Australian acacias in South Africa have been implemented and are aimed at suppressing reproductive vigour and, in some cases, vegetative growth of these weeds. Gall-forming midges are under consideration as potential biological control agents for invasive acacias in South Africa. Entomological surveys in southern Australia found a diverse cecidomyiid fauna associated with the buds, flowers and fruits of Acacia species. Nine new Dasineura species are described and two species, D. acaciaelongifoliae (Skuse) and D. dielsi Ru¨bsaamen, are redescribed. The newly described taxa are D. fistulosa sp.n., D. furcata sp.n., D. glauca sp.n., D. glomerata sp.n., D. oldfieldii sp.n., D. oshanesii sp.n., D. pilifera sp.n., D. rubiformis sp.n. and D. sulcata sp.n. All eleven species induce galls on ovaries and prevent the formation of fruit. Two general types of gall are caused. Type A comprises woody, tubular galls with larvae living inside ovaries (D. acaciaelongifoliae, D. dielsi, D. fistulosa, D. furcata, D. glauca, D. glomerata, D. oldfieldii). -

A New Dasineura Species (Diptera: Cecidomyiidae) Associated with Symplocos Cochinchinensis (Loureiro) (Symplocaceae) in Japan
Japanese Journal of Systematic Entomology, 23 (1): 81–86. June 15, 2017. A New Dasineura Species (Diptera: Cecidomyiidae) Associated with Symplocos cochinchinensis (Loureiro) (Symplocaceae) in Japan Ayman K. ELSAYED1,2), Koreyoshi OGATA3), Koichi KABURAGI4), Junichi YUKAWA5), and Makoto TOKUDA 1,6) 1) The United Graduate School of Agricultural Sciences, Kagoshima University, Kagoshima, 890-0065 Japan. Email: [email protected] 2) Department of Applied Entomology, Faculty of Agriculture, Alexandria University, Egypt 3) Nishino-omote, Nishino-omote City, Kagoshima, 891-3101 Japan. 4) Noma, Nakatane Town, Kagoshima, 891-3604 Japan 5) Entomological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka 812-8581, Japan. Email: [email protected] 6) Laboratory of Systems Ecology, Faculty of Agriculture, Saga University, Honjo 1, Saga 840-8502, Japan. Email: [email protected] Corresponding address: The United Graduate School of Agricultural Sciences, Kagoshima University, Kagoshima, 890-0065 Japan Abstract Recently, a gall midge induces leaf bud galls on Symplocos cochinchinensis (Loureiro) (Symplocaceae) was found on Tanegashima Island, Kagoshima, Japan. Based on morphological observation, the gall midge (Diptera: Cecidomyiidae) was clarified to be an undescribed species of the genus Dasineura Rondani (Lasiopteridi, Dasineurini). The species is distinguishable from other congeners by its unique undivided female 8th tergite, which is shorter than the 7th, in contrast with other Dasineura species possess longitudinally divided 8th tergite longer than the 7th. The new species, Dasineura symplocos Elsayed and Tokuda n. sp., is the first example of Dasineura species associated with Symplocaceae. Introduction During the course of our taxonomic and faunistic studies of gall midges in Japan, we found an undescribed species of gall The angiosperm plant family Symplocaceae consists of midge that induces axillary bud galls (Fig. -

CROP and PRAIRIE GRASSES SERVING AS HOSTS for the HESSIAN FLY MAYETIOLA DESTRUCTOR (SAY) (DIPTERA: CECIDOMYIIDAE) a Thesis Submi
CROP AND PRAIRIE GRASSES SERVING AS HOSTS FOR THE HESSIAN FLY MAYETIOLA DESTRUCTOR (SAY) (DIPTERA: CECIDOMYIIDAE) A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Yue Li In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Entomology November 2012 Fargo, North Dakota North Dakota State University Graduate School Title CROP AND PRAIRIE GRASSES SERVING AS HOSTS FOR THE HESSIAN FLY MAYETIOLA DESTRUCTOR (SAY) (DIPTERA: CECIDOMYIIDAE) By Yue Li The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of MASTER OF SCIENCE SUPERVISORY COMMITTEE: Marion Harris Chair Jason Harmon Steven Xu Steven Travers Approved: November 8, 2012 Frank Casey Date Department Chair ABSTRACT Insect herbivores typically parasitize a relatively small number of plant species. Host specialization is presumed to be a result of evolutionary arms races, with insect adaptations ultimately restricting host range. Being a gall-maker, the Hessian fly has highly evolved interactions with plant hosts. As a consequence, its host range is expected to be narrow. Two crop species, wheat and barley are hosts of the Hessian fly. I studied whether non-crop grasses can also serve as hosts. Included in tests were seven grass species that are important components of the grasslands of the Northern Great Plains. Although less suitable than wheat and barley, all seven species received eggs and five of the seven species supported development of offspring to the adult reproductive stage. Results indicate a broader host range than was expected. -

An Introduction to the Immature Stages of British Flies
Royal Entomological Society HANDBOOKS FOR THE IDENTIFICATION OF BRITISH INSECTS To purchase current handbooks and to download out-of-print parts visit: http://www.royensoc.co.uk/publications/index.htm This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.0 UK: England & Wales License. Copyright © Royal Entomological Society 2013 Handbooks for the Identification of British Insects Vol. 10, Part 14 AN INTRODUCTION TO THE IMMATURE STAGES OF BRITISH FLIES DIPTERA LARVAE, WITH NOTES ON EGGS, PUP ARIA AND PUPAE K. G. V. Smith ROYAL ENTOMOLOGICAL SOCIETY OF LONDON Handbooks for the Vol. 10, Part 14 Identification of British Insects Editors: W. R. Dolling & R. R. Askew AN INTRODUCTION TO THE IMMATURE STAGES OF BRITISH FLIES DIPTERA LARVAE, WITH NOTES ON EGGS, PUPARIA AND PUPAE By K. G. V. SMITH Department of Entomology British Museum (Natural History) London SW7 5BD 1989 ROYAL ENTOMOLOGICAL SOCIETY OF LONDON The aim of the Handbooks is to provide illustrated identification keys to the insects of Britain, together with concise morphological, biological and distributional information. Each handbook should serve both as an introduction to a particular group of insects and as an identification manual. Details of handbooks currently available can be obtained from Publications Sales, British Museum (Natural History), Cromwell Road, London SW7 5BD. Cover illustration: egg of Muscidae; larva (lateral) of Lonchaea (Lonchaeidae); floating puparium of Elgiva rufa (Panzer) (Sciomyzidae). To Vera, my wife, with thanks for sharing my interest in insects World List abbreviation: Handbk /dent. Br./nsects. © Royal Entomological Society of London, 1989 First published 1989 by the British Museum (Natural History), Cromwell Road, London SW7 5BD. -

Are Gall Midge Species (Diptera, Cecidomyiidae) Host-Plant Specialists? 367
Are gallAre midge gall species midge (Diptera, species Cecidomyiidae) (Diptera, host-plant Cecidomyiidae) specialists? host-plant specialists? 365 Marco Antonio A. Carneiro1, Cristina S. A. Branco2, Carlos E. D. Braga2, Emmanuel D. Almada2, Marina B. M. Costa2, Valéria C. Maia3 & Geraldo Wilson Fernandes2 1Laboratório Entomologia Ecológica, Universidade Federal de Ouro Preto, Campus Morro do Cruzeiro, 35400-000 Ouro Preto-MG, Brazil. [email protected] 2Ecologia Evolutiva & Biodiversidade, Universidade Federal de Minas Gerais; Caixa Postal 486, 30161-970 Belo Horizonte-MG, Brazil. [email protected], [email protected], [email protected], [email protected], [email protected] 3Museu Nacional, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro-RJ, Brazil. [email protected] ABSTRACT. Are gall midge species (Diptera, Cecidomyiidae) host plant specialists? Despite the speciose fauna of gall- inducing insects in the Neotropical region, little is known about their taxonomy. On the other hand, gall morphotypes associated with host species have been extensively used as a surrogate of the inducer species worldwide. This study reviewed the described gall midges and their galls to test the generalization on the use of gall morphotypes as surrogates of gall midge species in the Brazilian fauna. We compiled taxonomic and biological data for 196 gall midge species recorded on 128 host plant species. Ninety two percent of those species were monophagous, inducing galls on a single host plant species, whereas only 5.6% species were oligophagous, inducing galls on more than one congeneric host plant species. Only four species induced galls on more than one host plant genus. -
Diptera, Cecidomyiidae) Associated with Myrciaria Delicatula (Myrtaceae) from Brazil, with Identification Keys of Tribes and Unplaced Genera
Biota Neotrop., vol. 13, no. 2 A new genus and species of Lasiopteridi (Diptera, Cecidomyiidae) associated with Myrciaria delicatula (Myrtaceae) from Brazil, with identification keys of tribes and unplaced genera Alene Ramos Rodrigues1,3, Valéria Cid Maia1, Cristina Rodrigues Wenzel2 & Milton de Souza Mendonça Junior2 1Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro – UFRJ, Quinta da Boa Vista, s/n, São Cristóvão, CEP 20940-040, Rio de Janeiro, RJ, Brazil 2Departamento de Ecologia, Instituto de Biociências, Universidade Federal do Rio Grande do Sul – UFRGS, Av. Bento Gonçalves, 9500, CEP 91501-970, Porto Alegre, RS, Brazil 3Corresponding author: Alene Ramos Rodrigues, e-mail: [email protected] RODRIGUES, A.R., MAIA, V.C., WENZEL, C.R. & MENDONÇA JUNIOR, M.S. A new genus and species of Lasiopteridi (Diptera, Cecidomyiidae) associated with Myrciaria delicatula (Myrtaceae) from Brazil, with identification keys of tribes and unplaced genera.Biota Neotrop. (13)2: http://www.biotaneotropica.org. br/v13n2/en/abstract?identification-key+bn02213022013 Abstract: Fernandesia meridionalis Rodrigues & Maia, a new genus and species of Cecidomyiidae associated with Myrciaria delicatula O. Berg (Myrtaceae) is described and illustrated (male, female, pupa, and larva) based on material from Rio Grande do Sul, Brazil. The new genus belongs to Lasiopteridi, but it cannot be placed to tribe. A key to the tribes of Lasiopteridi and one to the unplaced genera of Neotropical Lasiopteridi are given. Keywords: Myrciaria, galler insect, taxonomy, Rio Grande do Sul. RODRIGUES, A.R., MAIA, V.C., WENZEL, C.R. & MENDONÇA JUNIOR, M.S. Um novo gênero e espécie de Lasiopteridi (Diptera, Cecidomyiidae) associado à Myrciaria delicatula (Myrtaceae) do Brasil, com chaves de identificação para tribos e gêneros não posicionados.