Preliminary Soil Remediation Goals (PSRG) Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Liquid Ammonium Polyphosphate As a Carrier of Iron and Zinc
EVALUATION OF LIQUID AMMONIUM POLYPHOSPHATE AS A CARRIER OF IRON AND ZINC by ; V<P RATMUNDO RALLAN GANIRON B. S. A., University of the Philippines, 1961 A MASTER'S THESIS submitted in partial fulfillment of the requirement for the degree MASTER OF SCIENCE Department of Agronomy KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: Jor Professor LP ZGGf it Tj( £/<?7 TABLE OF CONTENTS INTRODUCTION 1 REVIEW OF LITERATURE 3 Iron . 3 Zinc * MATERIALS AND METHODS 13 Fertiliser Materials 13 Field Experiments H Soil Sampling 20 Laaf Sampling 22 Chemical Analysis 22 (a) Zinc 22 (b) Iron 24 (c) Phosphorua 25 (d) Potassium •« 27 (a) Organic Matter 27 (f) pH 27 (g) Protain 27 RESULTS AND DISCUSSIONS 26 SUMMARY AND CONCLUSIONS 4$ ACKNOWLEDGMENT. 51 LITERATURE CITED 52 INTRODUCTION Increasing attention has baan focused to the need of fertilising with micronutrient elements to achieve maximum production and optimum quality of farm crops. The supply of these elements in the soil has become limited in some areas as a result of the introduction of high yielding varieties, better and more intensive cropping practices, and increased use of high analysis fertilisers. In some cases, the ob- served deficiencies of these micronutrients have been man- made due to addition of interfering elements for other pur- poses. For instance there is the copper induced iron chlorosis, copper being introduced as agricultural sprays and the phosphorus induced iron and zinc deficiencies, phos- phorus being introduced to meet the phosphorus requirement of farm crops. How to minimise such interactions and the problems of keeping these micronutrient elements available to the plants when applied to the soil have been the sub- ject of intensive researches. -

Effects of Different Sources of Fertilizer Nitrogen on Growth and Nutrition of Western Hemlock Seedlings
Effects of Different Sources U.S. Department of Agriculture Forest Service Pacific Northwest Forest of FertiIizer Nitrogen and Range Experiment Station Research Paper PNW-267 on Growth and Nutrition oJ February 1980 Western Hemlock Seedlings ---. --_. ------------------------ , I _J Authors M. A. RADWAN is Principal Plant Physiologist and DEAN S. DeBELL is Principal Silviculturist with the Forest Service, u.S. Department of Agriculture, Pacific Northwest Forest and Range Experiment Station, Forestry Sciences Laboratory, Olympia, Washington. En gl ish Equivalents 1 liter 0.2642 gallon 1 kilogram = 2.2046 pound 1 gram = 0.0353 ounce 1 centimeter = 0.3937 inch 1 kilogram per hectare 1.1206 pounds per acre (9/50C) + 32 = of EFFECTS OF DIFFERENT SOURCES OF FERTILIZER NITROGEN ON GROWTH AND NUTRITION OF WESTERN HEMLOCK Reference Abstract Radwan, M. A. , and Dean S. DeBell. 1980. Effects of different sources of fertilizer nitrogen on growth and nutrition of western hemlock seedlings. USDA For. Servo Res. Pap. PNW-267, 15 p. Pacific Northwest Forest and Range Experiment Station, Portland, Oregon. Twelve different nitrogen (N) fertilizer treatments were tested on potted western hemlock (Tsuga heterophylla (Raf. ) Sarg.) seedlings. Fertilizers affected soil N and pH, and growth and foliar chemical com position of seedlings. Ura plus N-Serve and sulfur-coated urea appear more promising for promoting growth than other fertilizers tested. Results, however, do not explain reported variability in response of hemlock stands to N fertilization. Keywords: Nitrogen fertilizer response, seedling growth, western hemlock, Tsuga heterophylla. RESEARCH SUMMARY Research Paper PNW-267 1980 The following fertilization treatments were applied in the spring to potted, 4-year-old western hemlock (Tsuga heterophylla (Raf. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -

United States Patent (19) 11 Patent Number: 5,788,915 Blount (45) Date of Patent: Aug
IIIUSOO5788915A United States Patent (19) 11 Patent Number: 5,788,915 Blount (45) Date of Patent: Aug. 4, 1998 54 FLAME RETARDANT COMPOSITIONS 57 ABSTRACT UTILIZING PARTIALLY HYDROLYZED AMNO CONDENSATON COMPOUNDS Flame retardant compositions of this invention are produced by incorporating a partially index (LOI) hydrolyzed amino 76 Inventor: David H. Blount, 6728 Del Cerro condensation composition in a more flammable organic Blvd., San Diego, Calif. 92120 material. The partially hydrolyzed amino condensation com pounds are produced by heating urea or heating urea with (21) Appl. No.: 801,776 other nitrogen containing compounds that will condensate with or react with isocyanic acid and/or cyanic acid thereby 22 Filed: Feb. 14, 1997 producing an amino condensation compound which is then Related U.S. Application Data partially hydrolysis is done by reacting it with a limited amount of water. The partially hydrolyzed amino conden 62) Division of Ser. No. 723,779, Sep. 30, 1996. sation compounds may be used alone or may be mixed with or reacted with carbonization auxiliaries, aldehydes and (51) Int. Cl. ........................ C09K 21A00; C08G 12/12 fillers to produce a partially hydrolyzed amino condensation 52 U.S. Cl. ........................... 252/609; 528/259; 252/601 composition which is incorporated in more flammable 58) Field of Search ..................................... 252/609, 601: organic compositions such as polyurethanes, polyester 528/259 resins. epoxy resins, vinyl resins and other resins. The partially hydrolyzed amino condensation salts of 56 References Cited phosphorus, boron or sulfur containing compounds and the U.S. PATENT DOCUMENTS partially hydrolyzed amino condensation-aldehyde resins 3,900,665 8/1975 Weil ....................................... -
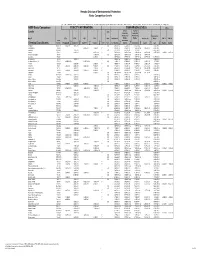
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

11-37-0 Liquid Ammonium Polyphosphate Guaranteed Analysis Total Nitrogen (N)
Product Data Sheet 11-37-0 LIQUID AMMONIUM POLYPHOSPHATE GUARANTEED ANALYSIS TOTAL NITROGEN (N) ................................................................................................................................ 11.00% 11.00% Ammoniacal Nitrogen AVAILABLE PHOSPHATE (P2O5) .................................................................................................................37.00% Molybdenum (Mo).........................................................................................................................................0.001% Derived from Ammonium Phosphate. KEEP OUT OF REACH OF CHILDREN tanks and certain fiberglass tanks. Aluminum tanks WARNING can also be used. However, aluminum tanks are not CAUSES SKIN IRRITATION. CAUSES EYE recommended for prolonged storage. IRRITATION. MAY CAUSE RESPIRATORY 2. Pumps: Any pump recommended for use with liquid IRRITATION. fertilizers. Remember the basic rule of "no brass." PRECAUTIONARY STATEMENTS: Avoid breathing fumes / gas / mist / vapors / spray. Wash thoroughly after handling. Use only USES outdoors or in a well-ventilated area. Call a poison center / doctor if 1. Is an excellent source of water soluble, fast acting you feel unwell. Specific treatment see First Aid section on this label. ammonium phosphate with the most desirable ratio of PERSONAL PROTECTIVE EQUIPMENT: Wear protective N:P2O5. gloves / protective clothing / eye protection / face protection. Take off 2. An ideal source of phosphate and nitrogen in complete contaminated clothing and wash before reuse. liquid mixes. FIRST AID: IF ON SKIN Wash with plenty of water. If skin irritation 3. For specific application rates follow the recommendation occurs, get medical advice / attention. IF INHALED Remove person of a qualified individual or institution, such as, but to fresh air and keep comfortable for breathing. IF IN EYES Rinse not limited to, a certified crop advisor, agronomist, cautiously with water for several minutes. Remove contact lenses, if university crop extension publication, or apply present and easy to do. -

United States Patent (19) (11) 3,904,558 Graham Et Al
United States Patent (19) (11) 3,904,558 Graham et al. (45) Sept. 9, 1975 54) PREPARATION OF LATEX FOAM Primary Examiner-Morton Foelak RUBBERS AND FILMS Attorney, Agent, or Firm-Stevens, Davis, Miller & 75 Inventors: Everett Steadman Graham; Ernest Mosher George Pole, both of Sarnia, Canada 73) Assignee: Polysar Limited, Sarnia, Canada 57 ABSTRACT An amine-sulfamate is added to a natural rubber latex (22) Filed: May 31, 1974 or a latex of a rubbery C-Co conjugated diene poly 21 Appl. No.: 474,962 mer in which the rubbery polymer particles are main tained in a dispersed state by an emulsifier which Related U.S. Application Data forms water-insoluble compounds on reaction with 63 Continuation-in-part of Ser. Nos. 372,502, June 22, zinc or cadmium ions to form a latex composition 1973, abandoned, and Ser. No. 372,521, June 22, which is suitable for use in the preparation of latex 1973, abandoned. foam rubbers and films. The composition is stable to storage at ambient temperatures for lengthy periods of 52 U.S. Cl...... 260/2.5 L; 260/2.5 H; 260/2.5 HB; time. To prepare the foam rubber, the amine-sulfa 260/4 R; 260/4 AR; 260/29.7 UA; 260/29.7 mate-containing latex is compounded with (1) a mate H; 260/723; 260/887; 260/890; 260/892; rial which supplies zinc or cadmium ions such as an 428/96; 428/315 oxide or carbonate of zinc or cadmium, (2) ammonia 51 Int. Cl... C08f 47/08; C08c 17/08; C08d 13/08 or a compound which releases ammonia on heating, 58) Field of Search... -

Ammonium Sulfamate
SAFETY DATA SHEET This safety data sheet was created pursuant to the requirements of: US OSHA Hazard Communication Standard (29 CFR 1910.1200) and Canada WHMIS 2015 which includes the amended Hazardous Products Act (HPA) and the Hazardous Products Regulation (HPR) Issuing Date 12-Dec-2019 Revision Date 30-Jul-2020 Revision Number 2 1. Identification Product identifier Product Name Ammonium Sulfamate Other means of identification Product Code(s) PN000173, PN002246 Recommended use of the chemical and restrictions on use Recommended use Laboratory reagent For professional use only Restrictions on use No information available. Details of the supplier of the safety data sheet Supplier Address Associates of Cape Cod, Inc. 124 Bernard E. Saint Jean Drive East Falmouth, MA 02536-4445 508 540 3444 E-mail [email protected] Emergency telephone number Emergency Telephone CHEMTREC: +1-703-527-3887 (INTERNATIONAL) 1-800-424-9300 (NORTH AMERICA) 2. Hazard(s) identification Classification Not classified. Label elements Hazard statements Not classified. Other information No information available. _____________________________________________________________________________________________ (M)SDS Number UL-ACC-015 R_REG_SDS_0189 Page 1 / 8 Ammonium Sulfamate Revision Date: 30-Jul-2020 _____________________________________________________________________________________________ 3. Composition/information on ingredients Substance Not applicable. Mixture Chemical name CAS No Weight-% Hazardous Material Date HMIRA filed and Information Review date exemption Act registry number granted (if (HMIRA registry #) applicable) Ammonium sulfamate 7773-06-0 1-4 - - 4. First-aid measures Description of first aid measures Inhalation Remove to fresh air. Eye contact Rinse thoroughly with plenty of water, also under the eyelids. Get medical attention if symptoms occur. Skin contact Wash skin with soap and water. -
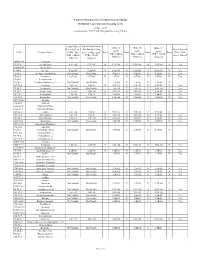
(GWSL) Ug/L TCR=1.0E-05 THQ=0.2 Sub-Slab and Exterior
North Carolina Department of Environmental Quality Residential Vapor Intrusion Screening Levels October 2017 (based on June 2017 USEPA Regional Screening Tables) Ground Water Sub-slab and Exterior IASL (A) IASL (B) IASL (C)3 Screening Level Soil Gas Screening 3 3 3 Does Chemical 3 ug/m ug/m ug/m CAS # Chemical Name: (GWSL) ug/L Level (SGSL) ug/m Basis Basis Basis Basis Have a Non- TCR=1.0E-06 TCR=1.0E-05 TCR=1.0E-04 4 TCR=1.0E-05 TCR=1.0E-05 Cancer Effect? THQ=0.2 THQ=0.2 THQ=0.2 THQ=0.2 THQ=0.2 30560-19-1 Acephate 75-07-0 Acetaldehyde 6.9E+02 6.3E+01 N 1.3E+00 C 1.9E+00 N 1.9E+00 N Yes 34256-82-1 Acetochlor 67-64-1 Acetone 4.5E+06 2.2E+05 N 6.5E+03 N 6.5E+03 N 6.5E+03 N Yes 75-86-5 Acetone Cyanohydrin Not Volatile Not Volatile 4.2E-01 N 4.2E-01 N 4.2E-01 N Yes 75-05-8 Acetonitrile 8.9E+03 4.2E+02 N 1.3E+01 N 1.3E+01 N 1.3E+01 N Yes 98-86-2 Acetophenone 53-96-3 Acetylaminofluorene, 2- Not Volatile Not Volatile 2.2E-03 C 2.2E-02 C 2.2E-01 C 107-02-8 Acrolein 8.4E-01 1.4E-01 N 4.2E-03 N 4.2E-03 N 4.2E-03 N Yes 79-06-1 Acrylamide Not Volatile Not Volatile 1.0E-02 C 1.0E-01 C 1.0E+00 C Yes 79-10-7 Acrylic Acid 1.4E+04 7.0E+00 N 2.1E-01 N 2.1E-01 N 2.1E-01 N Yes 107-13-1 Acrylonitrile 7.3E+01 1.4E+01 C 4.1E-02 C 4.1E-01 C 4.2E-01 N Yes 111-69-3 Adiponitrile Not Volatile Not Volatile 1.3E+00 N 1.3E+00 N 1.3E+00 N Yes 15972-60-8 Alachlor 116-06-3 Aldicarb 1646-88-4 Aldicarb Sulfone 1646-87-3 Aldicarb sulfoxide 309-00-2 Aldrin 3.2E+00 1.9E-01 C 5.7E-04 C 5.7E-03 C 5.7E-02 C 107-18-6 Allyl Alcohol 1.0E+02 7.0E-01 N 2.1E-02 N 2.1E-02 N 2.1E-02 -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Contents / Iii
FCC 11 Contents / iii Contents 1 I; ~ith ~er PREFACE v i I PEOPLE xi NEW AND CHANGED..................................................................................................................................................... xiii GENERAL PROVISIONS AND REQUIREMENTS APPLYING TO SPECIFICATIONS, TESTS, AND ASSAYS OF THE FOOD CHEMICALS CODEX .. MONOGRAPHS.................................................................................................................................................................. 9 should IDENTITY STANDARDS.............................................................................................................................................. 1269 GENERAL TESTS AND ASSAyS................................................................................................................................... 1275 Appendix I: Apparatus for Tests and Assays.............................................................................................................. 1279 Appendix II: Physical Tests and Determinations........................................................................................................ 1282 A. Chromatography......................... 1282 B. Physicochemical Properties......................................................................................................................... 1290 C. Others............................. 1302 Appendix III: Chemical Tests and Determinations....................................................................................................