Page 1 of 48 Ammonia in Workplace Atmospheres
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -
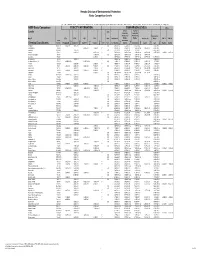
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

United States Patent (19) (11) 3,904,558 Graham Et Al
United States Patent (19) (11) 3,904,558 Graham et al. (45) Sept. 9, 1975 54) PREPARATION OF LATEX FOAM Primary Examiner-Morton Foelak RUBBERS AND FILMS Attorney, Agent, or Firm-Stevens, Davis, Miller & 75 Inventors: Everett Steadman Graham; Ernest Mosher George Pole, both of Sarnia, Canada 73) Assignee: Polysar Limited, Sarnia, Canada 57 ABSTRACT An amine-sulfamate is added to a natural rubber latex (22) Filed: May 31, 1974 or a latex of a rubbery C-Co conjugated diene poly 21 Appl. No.: 474,962 mer in which the rubbery polymer particles are main tained in a dispersed state by an emulsifier which Related U.S. Application Data forms water-insoluble compounds on reaction with 63 Continuation-in-part of Ser. Nos. 372,502, June 22, zinc or cadmium ions to form a latex composition 1973, abandoned, and Ser. No. 372,521, June 22, which is suitable for use in the preparation of latex 1973, abandoned. foam rubbers and films. The composition is stable to storage at ambient temperatures for lengthy periods of 52 U.S. Cl...... 260/2.5 L; 260/2.5 H; 260/2.5 HB; time. To prepare the foam rubber, the amine-sulfa 260/4 R; 260/4 AR; 260/29.7 UA; 260/29.7 mate-containing latex is compounded with (1) a mate H; 260/723; 260/887; 260/890; 260/892; rial which supplies zinc or cadmium ions such as an 428/96; 428/315 oxide or carbonate of zinc or cadmium, (2) ammonia 51 Int. Cl... C08f 47/08; C08c 17/08; C08d 13/08 or a compound which releases ammonia on heating, 58) Field of Search... -

Ammonium Sulfamate
SAFETY DATA SHEET This safety data sheet was created pursuant to the requirements of: US OSHA Hazard Communication Standard (29 CFR 1910.1200) and Canada WHMIS 2015 which includes the amended Hazardous Products Act (HPA) and the Hazardous Products Regulation (HPR) Issuing Date 12-Dec-2019 Revision Date 30-Jul-2020 Revision Number 2 1. Identification Product identifier Product Name Ammonium Sulfamate Other means of identification Product Code(s) PN000173, PN002246 Recommended use of the chemical and restrictions on use Recommended use Laboratory reagent For professional use only Restrictions on use No information available. Details of the supplier of the safety data sheet Supplier Address Associates of Cape Cod, Inc. 124 Bernard E. Saint Jean Drive East Falmouth, MA 02536-4445 508 540 3444 E-mail [email protected] Emergency telephone number Emergency Telephone CHEMTREC: +1-703-527-3887 (INTERNATIONAL) 1-800-424-9300 (NORTH AMERICA) 2. Hazard(s) identification Classification Not classified. Label elements Hazard statements Not classified. Other information No information available. _____________________________________________________________________________________________ (M)SDS Number UL-ACC-015 R_REG_SDS_0189 Page 1 / 8 Ammonium Sulfamate Revision Date: 30-Jul-2020 _____________________________________________________________________________________________ 3. Composition/information on ingredients Substance Not applicable. Mixture Chemical name CAS No Weight-% Hazardous Material Date HMIRA filed and Information Review date exemption Act registry number granted (if (HMIRA registry #) applicable) Ammonium sulfamate 7773-06-0 1-4 - - 4. First-aid measures Description of first aid measures Inhalation Remove to fresh air. Eye contact Rinse thoroughly with plenty of water, also under the eyelids. Get medical attention if symptoms occur. Skin contact Wash skin with soap and water. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Exhibit 2D-3
Exhibit 2D–3. Hazardous Substances 1. Acetaldehyde 73. Captan 144. Ferrous sulfate 2. Acetic acid 74. Carbaryl 145. Formaldehyde 3. Acetic anhydride 75. Carbofuran 146. Formic acid 4. Acetone cyanohydrin 76. Carbon disulfide 147. Fumaric acid 5. Acetyl bromide 77. Carbon tetrachloride 148. Furfural 6. Acetyl chloride 78. Chlordane 149. Guthion 7. Acrolein 79. Chlorine 150. Heptachlor 8. Acrylonitrile 80. Chlorobenzene 151. Hexachlorocyclopentadiene 9. Adipic acid 81. Chloroform 152. Hydrochloric acid 10. Aldrin 82. Chloropyrifos 153. Hydrofluoric acid 11. Allyl alcohol 83. Chlorosulfonic acid 154. Hydrogen cyanide 12. Allyl chloride 84. Chromic acetate 155. Hydrogen sulfide 13. Aluminum sulfate 85. Chromic acid 156. Isoprene 14. Ammonia 86. Chromic sulfate 157. Isopropanolamine dodecylbenzenesulfonate 15. Ammonium acetate 87. Chromous chloride 158. Kelthane 16. Ammonium benzoate 88. Cobaltous bromide 159. Kepone 17. Ammonium bicarbonate 89. Cobaltous formate 160. Lead acetate 18. Ammonium bichromate 90. Cobaltous sulfamate 161. Lead arsenate 19. Ammonium bifluoride 91. Coumaphos 162. Lead chloride 20. Ammonium bisulfite 92. Cresol 163. Lead fluoborate 21. Ammonium carbamate 93. Crotonaldehyde 164. Lead fluorite 22. Ammonium carbonate 94. Cupric acetate 165. Lead iodide 23. Ammonium chloride 95. Cupric acetoarsenite 166. Lead nitrate 24. Ammonium chromate 96. Cupric chloride 167. Lead stearate 25. Ammonium citrate 97. Cupric nitrate 168. Lead sulfate 26. Ammonium fluoroborate 98. Cupric oxalate 169. Lead sulfide 27. Ammonium fluoride 99. Cupric sulfate 170. Lead thiocyanate 28. Ammonium hydroxide 100. Cupric sulfate ammoniated 171. Lindane 29. Ammonium oxalate 101. Cupric tartrate 172. Lithium chromate 30. Ammonium silicofluoride 102. Cyanogen chloride 173. Malathion 31. Ammonium sulfamate 103. Cyclohexane 174. Maleic acid 32. Ammonium sulfide 104. -

Ammonium Sulfamate Hazard Summary Identification
Common Name: AMMONIUM SULFAMATE CAS Number: 7773-06-0 RTK Substance number: 0114 DOT Number: NA 9089 Date: December 1994 Revision: January 2001 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Ammonium Sulfamate can affect you when breathed in. OSHA: The legal airborne permissible exposure limit * Contact can cause skin and eye irritation. (PEL) is 15 mg/m3 (for total dust) and 5 mg/m3 * Breathing Ammonium Sulfamate can irritate the nose (for respirable dust) averaged over an 8-hour and throat causing coughing and/or shortness of breath. workshift. * Exposure to very high levels may cause nausea and vomiting. NIOSH: The recommended airborne exposure limit is 10 mg/m3 (for total dust) and 5 mg/m3 (for IDENTIFICATION respirable dust) averaged over a 10-hour Ammonium Sulfamate is a white crystalline (sand-like) workshift. solid. It is used to flameproof textile products and paper, and as a weed killer. ACGIH: The recommended airborne exposure limit is 10 mg/m3 averaged over an 8-hour workshift. REASON FOR CITATION * Ammonium Sulfamate is on the Hazardous Substance WAYS OF REDUCING EXPOSURE List because it is regulated by OSHA and cited by * Where possible, enclose operations and use local exhaust ACGIH, DOT, NIOSH, HHAG and EPA. ventilation at the site of chemical release. If local exhaust * Definitions are provided on page 5. ventilation or enclosure is not used, respirators should be worn. HOW TO DETERMINE IF YOU ARE BEING * Wear protective work clothing. EXPOSED * Wash thoroughly immediately after exposure to The New Jersey Right to Know Act requires most employers Ammonium Sulfamate. to label chemicals in the workplace and requires public * Post hazard and warning information in the work area. -

SWPPP Appendix E Hazmat
Appendix E. Oil and Hazardous Materials Material Category RQ in Reporting Requirements pounds (kilograms) In the event of a spill of oil that reaches any surface Ammonia B 100 (45.4) waters, or a spill on land of certain hazardous substances Ammonium acetate D 5,000 (2,270) (listed on the following pages) exceeding the Reportable Ammonium benzoate D 5,000 (2,270) Quantity (RQ) level, the Contractor must: Ammonium bicarbonate D 5,000 (2,270) Ammonium bichromate A 10 (4.54) • Notify the Project Engineer Ammonium bifluoride B 100 (45.4) • Notify the National Response Center in Ammonium bisulfite D 5,000 (2,270) Washington, D.C., immediately at (800) 424- Ammonium carbamate D 5,000 (2,270) 8802 Ammonium carbonate D 5,000 (2,270) • Within 14 days, submit a written description of Ammonium chloride D 5,000 (2,270) the release to the EPA regional office providing Ammonium chromate A 10 (4.54) the date and circumstances of the release and the Ammonium citrate dibasic D 5,000 (2,270) steps to be taken to prevent another release. Ammonium fluoborate D 5,000 (2,270) • Modify the SWPPP to include the information Ammonium fluoride B 100 (45.4) listed above. Ammonium hydroxide C 1,000 (454) • Notify the Alaska Department of Environmental Ammonium oxalate D 5,000 (2,270) Conservation (ADEC) at one of the following Ammonium silicofluoride C 1,000 (454) telephone numbers, depending upon project Ammonium sulfamate D 5,000 (2,270) location: Ammonium sulfide B 100 (45.4) o Central (Anchorage) 907-269-3063 Ammonium sulfite D 5,000 (2,270) o Northern (Fairbanks) 907-451-2121 Ammonium tartrate D 5,000 (2,270) o Southeast (Juneau) 907-465-5340 Ammonium thiocyanate D 5,000 (2,270) o Outside normal business hours, call: Amyl acetate D 5,000 (2,270) 1-800-478-9300 Aniline D 5,000 (2,270) • Complete an Oil and Hazardous Substances Spill Antimony pentachloride C 1,000 (454) Form and submit it to ADEC after telephone Antimony potassium B 100 (45.4) notification. -

Preliminary Soil Remediation Goals (PSRG) Table
North Carolina Department of Environmental Quality Preliminary Soil Remediation Goals (PSRGs) June 2021 (based on May 2021 USEPA Regional Screening Tables) These notes must be used with the PSRG Table General Notes: 1. PSRGs are obtained using the USEPA Regional Screening Level (RSL) table. If a contaminant does not have a PSRG listed, then one or more of the contaminant-specific parameters are not available from the RSL table to calculate a soil PSRG (these contaminants are only included in the PSRG table to account for all entries on the RSL table). If a 02L Standard or IMAC is available for a contaminant with no PSRGs, protection of groundwater evaluation is still necessary. Leaching tests should be performed by an analytical laboratory to determine whether this contaminant it is leaching to groundwater above its NC 02L Standard or IMAC, or another method may be allowed by the state program providing oversight. 2. The health-based PSRGs (Residential and Industrial/Commercial) are based upon human health risk and do not address potential ecological risk. The PSRGs listed are the lower of: a. the carcinogenic target risk of 1.0E-06 (C), or b. the non-carcinogenic target hazard quotient of 0.2 (N). Residential health-based PSRGs should be used to identify site contaminants and delineate their extent. 3. The protection of groundwater PSRGs are provided as a conservative indicator of soil leachability and are developed using a USEPA soil leaching model with conservative assumptions and default values appropriate for North Carolina (see Equation 1 on page 4). Check remedial program guidance or the Technical Guidance for Risk-Based Environmental Remediation of Sites published on the NCDEQ Risk-Based Remediation website for alternative methods to further characterize site-specific soil leachability if soil concentrations exceed protection of groundwater PSRGs. -

WO 2015/115962 Al 6 August 2015 (06.08.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/115962 Al 6 August 2015 (06.08.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C06B 25/34 (2006.01) C01B 21/082 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/SE20 15/000004 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 29 January 2015 (29.01 .2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: Swedish PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 1400043-4 30 January 2014 (30.01.2014) SE (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: TOTALFORSVARETS FORSKNINGSIN- GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, STITUT [SE/SE]; S-164 90 (SE). -

Reporting Requirements for Hazardous Substances and List of Hazardous Substances
1301:7-9-03 Reporting requirements for hazardous substances and list of hazardous substances. (A) Purpose. For the purpose of prescribing rules pursuant to section 3737.88 of the Revised Code, the fire marshal hereby adopts this rule to establish reporting requirements for underground storage tank systems that contain hazardous substance(s) and to list those substances which are hereby identified as hazardous substances. This rule is adopted by the fire marshal in accordance with Chapter 119 of the Revised Code and shall not be considered a part of the "Ohio Fire Code". (B) Definitions. For the purpose of this rule: (1) "Release of a hazardous substance" means: (a) Any spilling, leaking, emitting, discharging, escaping, leaching or disposing of a hazardous substance(s) from an underground storage tank system into the ground water, a surface water body, subsurface soils or otherwise into the environment; (b) Any spilling, leaking, emitting, discharging, escaping, or disposing of a hazardous substance(s) into ground water, a surface water body, subsurface soils or otherwise into the environment while transferring or attempting to transfer a hazardous substance(s) into an underground storage tank system; or (c) Contamination of subsurface soils or ground water on the UST site by a hazardous substance(s) found and confirmed through laboratory analysis of samples from the UST site. (2) "Suspected release of a hazardous substance" means evidence of a release of a hazardous substance(s) obtained through one or more of the following events: (a)