Industrial Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRICELIST-1920-FINAL.Pdf
INDEX Page No. MD Speech 01 Our Vision / Our Mission 02 Product Classification and Grade Information 03 Label Information 04 GHS Compliance 05 Technical Data Sheet and COA 06 Qualikems Product Range 07 ISO Certificate 08 - 09 Company Details 10 Ordering Information 11 Terms & Conditions 12 Rate List 13 - 52 Images of Lab / Plant / R & D 53 - 58 Rate List 59 -116 BELIEVING yourselfIN IS THE FIRST SECRET TO Success Dear Reader, The document you are holding is the result of work performed by the team of professionals of QUALIKEMS. It is the fruit of our teams extensive technical experience combine with the collaboration of our customers, who have offered us their valuable comments and proposals for improvement. At Qualikems, we have been working and investing for many years with our thoughts focused on the long term. Only thus can this comprehensive catalogue be kept up to date with the products you need. Our highly trained workforce, using state of the art technology, is the driving force behind the management of our modern factory, and our principal aim is to guarantee that the QUALIKEMS product range meets the conditions you require. QUALIKEMS reinforces industrial character and the path to progress we have continuously forged over the years. This path requires the responsible use of resources and the sustainability of our business activity. It is likewise requires and ability to keep on growing as the way to earn and to preserve our status as the leading supplier of laboratory reagents to our Clients Ashok Sahni Managing Director QUALIKEMS FINE CHEM PVT. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (iii) Which are used or could be used (j) Process waste water means any for industrial purposes by industries in water which, during manufacturing or interstate commerce; processing, comes into direct contact (4) All impoundments of waters oth- with or results from the production or erwise defined as navigable waters use of any raw material, intermediate under this paragraph; product, finished product, byproduct, (5) Tributaries of waters identified in or waste product. paragraphs (i) (1) through (4) of this section, including adjacent wetlands; [44 FR 50776, Aug. 29, 1979, as amended at 58 and FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, (6) Wetlands adjacent to waters iden- 2000] tified in paragraphs (i) (1) through (5) of this section (‘‘Wetlands’’ means § 117.2 Abbreviations. those areas that are inundated or satu- NPDES equals National Pollutant rated by surface or ground water at a Discharge Elimination System. RQ frequency and duration sufficient to equals reportable quantity. support, and that under normal cir- cumstances do support, a prevalence of § 117.3 Determination of reportable vegetation typically adapted for life in quantities. saturated soil conditions. Wetlands Each substance in Table 117.3 that is generally included playa lakes, listed in Table 302.4, 40 CFR part 302, is swamps, marshes, bogs, and similar assigned the reportable quantity listed areas such as sloughs, prairie potholes, in Table 302.4 for that substance. wet meadows, prairie river overflows, mudflats, and natural ponds): Provided, TABLE 117.3—REPORTABLE QUANTITIES That waste treatment systems (other OF HAZARDOUS SUBSTANCES DES- than cooling ponds meeting the cri- IGNATED PURSUANT TO SECTION 311 OF teria of this paragraph) are not waters THE CLEAN WATER ACT of the United States. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -

Hydrothermal Synthesis of Molybdenum Based Oxides for The
Hydrothermal synthesis of molybdenum based oxides for the application in catalysis Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) - Universitätsbereich genehmigte DISSERTATION von Dipl.-Ing. (FH) Kirsten Schuh aus Mainz Dekan: Prof. Dr. Peter Roesky Referent: Prof. Dr. Jan-Dierk Grunwaldt Korreferent: Prof. Dr. Anker Degn Jensen Tag der mündlichen Prüfung: 17. April 2014 Acknowledgements Acknowledgements I owe many thanks to a lot of people who have helped, supported and encouraged me during my doctoral studies, not just scientifically but also personally. First I would like to thank my supervisor Prof. Dr. Jan-Dierk Grunwaldt for the opportunity to complete my doctoral studies in his group and for providing me with a very interesting and diversified topic. I am grateful for the scientific freedom he gave me, the possibility to spend several months at the Technical University of Denmark as well as University of Zurich and for the opportunity to attend international conferences. I am grateful to Dr. Wolfgang Kleist for his scientific help especially with presentations and publications making the manuscripts reader friendly. I would also like to thank Prof. Dr. Anker Degn Jensen for agreeing to be my co- supervisor, for very helpful corrections and suggestions of abstracts, manuscripts and presentations and for giving me the opportunity to spend four months in his group at the Technical University of Denmark (DTU), where I felt very welcome. I am especially grateful for the help of Dr. Martin Høj, who put the selective oxidation set- up at DTU into operation, tested several of my samples for selective oxidation of propylene and performed TEM measurements of my FSP samples. -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

Chemical Products
CUSTOM MANUFACTURING AND FINE CHEMICAL SOURCING 768 N. Bethlehem Pike ⚫ Lower Gwynedd, PA 19002 USA Tel: (215) 628-2946 ⚫ Fax: (215) 628-4262 ⚫ Web: www.richmanchemical.com Chemical Products This is a representative list of products which Richman Chemical Inc. supplied, sourced for our customers, or custom manufactured. This partial list shows only what we have done in the past, and it may not include what we are capable of doing for you. Therefore, if you are looking for a specific material not on our list, please call with your inquiry. Satisfying your unique or special needs is our full time business. -
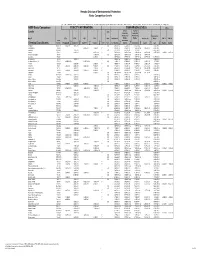
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

API Phosphate Test Kit Solution 1 Safety Data Sheet.Pdf
Phosphate Test Solution #1 Mars Fishcare North America, Inc. Chemwatch Hazard Alert Code: 4 Chemwatch: 4650-10 Issue Date: 11/16/2017 Version No: 6.1.1.1 Print Date: 10/23/2018 Safety Data Sheet according to OSHA HazCom Standard (2012) requirements S.GHS.USA.EN SECTION 1 IDENTIFICATION Product Identifier Product name Phosphate Test Solution #1 Synonyms Solution ID# 3352 Proper shipping name Battery fluid, acid; Sulfuric acid with not more than 51% acid Other means of Not Available identification Recommended use of the chemical and restrictions on use Relevant identified uses Phosphate test solution for product 63L. Name, address, and telephone number of the chemical manufacturer, importer, or other responsible party Registered company Mars Fishcare North America, Inc. name Address 50 E. Hamilton Street United States Telephone 215 822 8181 Fax 215 997 1290 Website Not Available Email Not Available Emergency phone number Association / Not Available Organisation Emergency telephone Not Available numbers Other emergency Not Available telephone numbers SECTION 2 HAZARD(S) IDENTIFICATION Classification of the substance or mixture NFPA 704 diamond Note: The hazard category numbers found in GHS classification in section 2 of this SDSs are NOT to be used to fill in the NFPA 704 diamond. Blue = Health Red = Fire Yellow = Reactivity White = Special (Oxidizer or water reactive substances) Metal Corrosion Category 1, Skin Corrosion/Irritation Category 1A, Serious Eye Damage Category 1, Skin Sensitizer Classification Category 1, Specific target organ toxicity - repeated exposure Category 2 Label elements Hazard pictogram(s) SIGNAL WORD DANGER Hazard statement(s) H290 May be corrosive to metals. H314 Causes severe skin burns and eye damage. -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

Lab Chemicals.Cdr
KIMYA Lab Chemicals Product Catalogue PRODUCTS Ammonium iron(ıı)sulfate hexa. Ammonium monovanadat 100 gr 1-Naphthylamine for synthesis Ammonium oxalate monoh.ext.pu 2-Butanol for analysis Ammonium peroxidısulfate ex.pu 2-Butanol gr for analysis Ammonium peroxidısulfate ex.pu 2-Mercaptoethanol Ammonium standart Acetaldehyde for synthesis Ammonium sulfate Acetic acid %100 gr for analysis Ammonium sulfate Acetic acid %100 gr for analysis pls. Ammonium thiocyanate gr for Acetic acid %100 extra pure Ammonium thıocyanate sol.for Acetic acid %100 extra pure Amonnium heptamolybdate Acetic acid (glacial) 100% extra pure Amyl alcohol Aceton gr for analysis acs,iso,reag,ph eur Amyl alcohol (1-pentanol)for.. Aceton gr for analysis acs,iso,reag,ph eur Aniline gr for analysis Aceton extra pure ph eur bp,nf Anthrone for synthesis Aceton extra pure ph eurbp,nf Antimony icp standart traceable to srm Acetone suprasolv 2,5 lt Antimony(ııı)chloride Acetone for liquid chromotography Aquamerck iron fresh-sea wa.te Acetonitrile for gas chromotography Aquamerck total hardness test Acetonitrile hplc Aqumerck total hardness test Acetonitrile hypergrade Arsenic icp standart traceable Acetonitrile lichrosolv Arsenic standard sol.1000mg/l Acetylacetone for synthesıs Azomethine h gr for analysis Acrylamide for electrophoresıs Barbituric acıd for synthesis Acrylamide for synthesis Barbituric acıd gr Acrylamıde for synthesis Barium chloride dıhydrate gr for analysis acs,iso Adipic acid for synthesis Barium chloride dihdrate ex.pu Albumin fraction v bovine seru Barium -

United States Patent (19) (11) 3,904,558 Graham Et Al
United States Patent (19) (11) 3,904,558 Graham et al. (45) Sept. 9, 1975 54) PREPARATION OF LATEX FOAM Primary Examiner-Morton Foelak RUBBERS AND FILMS Attorney, Agent, or Firm-Stevens, Davis, Miller & 75 Inventors: Everett Steadman Graham; Ernest Mosher George Pole, both of Sarnia, Canada 73) Assignee: Polysar Limited, Sarnia, Canada 57 ABSTRACT An amine-sulfamate is added to a natural rubber latex (22) Filed: May 31, 1974 or a latex of a rubbery C-Co conjugated diene poly 21 Appl. No.: 474,962 mer in which the rubbery polymer particles are main tained in a dispersed state by an emulsifier which Related U.S. Application Data forms water-insoluble compounds on reaction with 63 Continuation-in-part of Ser. Nos. 372,502, June 22, zinc or cadmium ions to form a latex composition 1973, abandoned, and Ser. No. 372,521, June 22, which is suitable for use in the preparation of latex 1973, abandoned. foam rubbers and films. The composition is stable to storage at ambient temperatures for lengthy periods of 52 U.S. Cl...... 260/2.5 L; 260/2.5 H; 260/2.5 HB; time. To prepare the foam rubber, the amine-sulfa 260/4 R; 260/4 AR; 260/29.7 UA; 260/29.7 mate-containing latex is compounded with (1) a mate H; 260/723; 260/887; 260/890; 260/892; rial which supplies zinc or cadmium ions such as an 428/96; 428/315 oxide or carbonate of zinc or cadmium, (2) ammonia 51 Int. Cl... C08f 47/08; C08c 17/08; C08d 13/08 or a compound which releases ammonia on heating, 58) Field of Search...