Minnesota Rules 2017, Part 5206.0400
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent ‘ Patented Mar
r 2,786,869 United States Patent ‘ Patented Mar. 26, 1957 l 2 tically, mixtures of tert-alkylamine such as are available on the market. Typical mixtures are those containing ‘ 2,786,869 C12H25NH2 to C15H31NH2 or C18H3'INH2 to C24H49NH2 N-TRIALKYLCARBINYL-N-(HYDROXYETHYL or C15H31- to C24H49NH2. These may be represented by POLYOXYETHYL) GLYCINES the formula Peter L. de Benneville and Homer J. Sims, Philadelphia, 31 Pa., assignors to Rohrn & Haas Company, Philadelphia, R’-—-C—NH2 Pa., a corporation of Delaware Rs No Drawing. Application June 16, 1954, 10 As catalysts in the ?rst step of the process of this Serial No. 437,273 invention, wherein the hydroxyethyl group is introduced, 9 Claims.‘ (or. 260-534) there may be used any of the strong acids, such as hy drochloric, hydrobromic, sulfuric, arylsulfonic, alkanesul fonic, or phosphoric. The preferred amount of this cat This invention relatesto-compounds of the structure 15 alyst is 10 to 30 mole percent of the amine. With R1 '(CH2CH2O),.H ‘ , amines from 12 carbon atoms upward it is exceedingly di?icult to introduce more than one hydroxyethyl group in a tert-alkylamine molecule. Such amines‘yicld ?nal 3 \CHrCOOH products which have the desired balance of properties. ' wherein R1, R2, and R3 are alkyl groups containing a 20 The ?rst reaction with ethylene oxide is effected by total of 11 to 23 carbon atoms and n is an integer having bringing together ethylene oxide and tert-alkylamine, a value from 5 to about 50 or more, preferably 5 to 25. usually by passing ethylene oxide into amine and catalyst, These compounds may be called N-(trialkylcarbinyl)-N at temperatures from 0° to 180° C. -

Hydroxyacetonitrile (HOCH2CN) As a Precursor for Formylcyanide (CHOCN), Ketenimine (CH2CNH), and Cyanogen (NCCN) in Astrophysical Conditions
A&A 549, A93 (2013) Astronomy DOI: 10.1051/0004-6361/201219779 & c ESO 2013 Astrophysics Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions G. Danger1, F. Duvernay1, P. Theulé1, F. Borget1, J.-C. Guillemin2, and T. Chiavassa1 1 Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397 Marseille, France e-mail: [email protected] 2 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France Received 8 June 2012 / Accepted 19 November 2012 ABSTRACT Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis. Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice. Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -

United States Patent Office Patented Jan
2,700,668 United States Patent Office Patented Jan. 25, 1955 1. 2 Glycolonitrile, as is known, is readily prepared by re acting formaldehyde and hydrogen cyanide. Cf. Prepa 2,700,668 ration of Formaldehyde Cyanohydrin, p. 168 in "Prepara PREPARATION OF KETOPPERAZINES tion of Organic Intermediates' by Shirley, John Wiley & 5 Sons, New York, 1951. See also, p. 176 of "Chemistry James S. Strong and W. E. Craig, Philadelphia, and Vin of Organic Cyanogen Compounds,” Migridichian, 1947 cent T. Elkind, Roslyn, Pa., assignors to Rohm & Haas ed. Glycolonitrile may also be prepared in the reaction Company, Philadelphia, Pa., a corporation of Delaware mixture as described in the next example wherein form No Drawing. Application February 8, 1952, aldehyde and hydrogen cyanide are reacted in the pres Serial No. 270,761 O ence of an amine catalyst. 7 Claims. (C. 260-268) Example 2 To 83 parts of a 36.3% aqueous formaldehyde solution there were added a trace of piperidine to serve as catalyst This invention concerns a method for preparing keto 5 and 27 parts of hydrogen cyanide. During addition of piperazines having the formula the hydrogen cyanide the temperature was kept between N. R. 27 and 30° C. by cooling. After the cyanide had been added, the temperature of the mixture was allowed to, cá, Yo4R, rise to 50° C. The mixture was allowed to stand for bH, d-o 20 3.5 hours. This solution was then slowly added to 78 N parts of 76.7% ethylenediamine in water, which was stirred and held at 100° C. -
Basic Comparison Levels
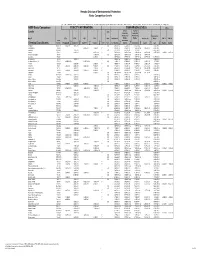
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

Lllllllllliillilllllllllllliihiillllllllllllllllllllllllll
llllllllllIIllIllllllllllllIIHIIllllllllllllllllllllllllllllllllllllllllll . US005208363A United States Patent [19] [11] Patent Number: 5,208,363 Crump et al. [45] Date of Patent: May 4, 1993 [54] PREPARATION OF AMINONITRILES 3,907,858 9/1975 Davis et a1. ....................... .. 558/346 3,925,448 12/1975 Tanier ................... .. 558/346 [75] Inv9nt9r$= B11199 K- Crumm Lake Jackson; 3,959,342 5/1976 Homberg et a1. ......... .. 558/346 Band A. W1lson, R1chwood, both of 3,988,360 10/1976 Gaudette et a1. .. 558/346 Tex. 4,022,815 5/1977 Schlecht 61 a1. 558/346 . 4,478,759 10/1984 Diatler C181. .. .558/346 [73] Asslgnee‘ “F D" @e'mcal Cmnpmy, 4,560,516 12/1985 Singer .......... ..' 558/346 M1d1and,M1¢h- 4,661,614 4/1987 M08161 61. .. 558/346 . 4,704,465 11/1987 Tannet et 111. ........ .. 558/346 [21] Appl' N°" 878’572 4,980,471 12/1990 Christiansen e181 ............ .. 544/384 [ 221 Fil e d: M “y 5’ 1992 OTHER PUBLICATIONS Related US. Application Data Stewart, et 81., J. Chem. Soc., pp. 3281-3285, (1940), [ 63 1 553333,?". _.Ma" _ “S” _ N° _ 597 , 625 , 0°‘ _ 15 , 199°’ V01.ChOh-HQO 62. Li, et aL, J. Chem. Soc., pp. 2596-2599, (1937), V01. 59. Céi5 .......................................... .. C07C5§33é2 Stewart, et 31“ J_ chem Soc” pp_ 27824737, (1933), n a O - . 6 . 6 3 . 6 . 0. vol. 60' I [58] Field of Search ....................................... .. 558/ 346 [56]‘ R f Cit‘ d Primary Examiner-Joseph Paul Brust e erences e U.s. PATENT DOCUMENTS :2] _ ‘ABS-“MCI f _ , e present 1nvent1on 1s a process or preparing 2,205,995 6/1940 Ulnch e181. -

United States Patent (19) (11) 3,904,558 Graham Et Al
United States Patent (19) (11) 3,904,558 Graham et al. (45) Sept. 9, 1975 54) PREPARATION OF LATEX FOAM Primary Examiner-Morton Foelak RUBBERS AND FILMS Attorney, Agent, or Firm-Stevens, Davis, Miller & 75 Inventors: Everett Steadman Graham; Ernest Mosher George Pole, both of Sarnia, Canada 73) Assignee: Polysar Limited, Sarnia, Canada 57 ABSTRACT An amine-sulfamate is added to a natural rubber latex (22) Filed: May 31, 1974 or a latex of a rubbery C-Co conjugated diene poly 21 Appl. No.: 474,962 mer in which the rubbery polymer particles are main tained in a dispersed state by an emulsifier which Related U.S. Application Data forms water-insoluble compounds on reaction with 63 Continuation-in-part of Ser. Nos. 372,502, June 22, zinc or cadmium ions to form a latex composition 1973, abandoned, and Ser. No. 372,521, June 22, which is suitable for use in the preparation of latex 1973, abandoned. foam rubbers and films. The composition is stable to storage at ambient temperatures for lengthy periods of 52 U.S. Cl...... 260/2.5 L; 260/2.5 H; 260/2.5 HB; time. To prepare the foam rubber, the amine-sulfa 260/4 R; 260/4 AR; 260/29.7 UA; 260/29.7 mate-containing latex is compounded with (1) a mate H; 260/723; 260/887; 260/890; 260/892; rial which supplies zinc or cadmium ions such as an 428/96; 428/315 oxide or carbonate of zinc or cadmium, (2) ammonia 51 Int. Cl... C08f 47/08; C08c 17/08; C08d 13/08 or a compound which releases ammonia on heating, 58) Field of Search... -

(2) Appl. No. 97.27 OTHER PUBLICATIONS 22) Filed: Oct
III US005187301A United States Patent 19) 11 Patent Number: 5,187,3019 9 Cullen et al. (45) Date of Patent: k Feb. 16, 1993 54 PREPARATION OF 3,412,137 11/1968 Stutts et al. ...................... 260/465.5 IMINODIACETONITRILE FROM 3,904,668 9/1975 Gaudette et al. ... 260/465.5. A GLYCOLONTRILE 3,988,360 10/1976 Gaudette et al. ............ 260/465.5. A 4,543,215 9/1985 Brunnmueller et al. ......... 260/465.5 75 Inventors: Barry A. Cullen, Lyndeboro; Brian 4,661,614 4/1987 Most et al. .......................... 558/346 A. Parker, Nashua, both of N.H. 4,895,971 1/1990 Su et al. .............................. 558/346 4,948,909 8/1990 K t al. ....................... 558/346 73) Assignee: W. R. Grace & Co.-Conn., New York, / oenge N.Y. FOREIGN PATENT DOCUMENTS * Notice: The portion of the term of this patent 3242.193 5/1984 Fed. Rep. of Germany . subsequent- - - to Jul. 16, 2009 has been o: 2. 3: Eleapan . disclaimed. 744675 2/1956 United Kingdom . (2) Appl. No. 97.27 OTHER PUBLICATIONS 22) Filed: Oct. 11, 1990 Eschweiler, Ann 278 (1894) pp. 232-243 (Translation of Related U.S. Application Data pp. 235-238 included). (63) continuation-in-partabandoned. of ser. No. 427,414, Oct. 26, 1989, Attorney,Primary ExaminerAgent, or Firm--KevinJoseph Paul S.Brust Lemack; William L. Baker 51) int. Cl. ............................................ C07C 253/30 52 U.S. C. ..................................... 558/.455; 558/346 (57) ABSTRACT 58) Field of Search ................................ 558/346,455 A process for producing iminodiacetonitrile (IDAN) 56) References Cited from glycolonitrile and ammonia or its salt is disclosed. -

Ammonium Sulfamate
SAFETY DATA SHEET This safety data sheet was created pursuant to the requirements of: US OSHA Hazard Communication Standard (29 CFR 1910.1200) and Canada WHMIS 2015 which includes the amended Hazardous Products Act (HPA) and the Hazardous Products Regulation (HPR) Issuing Date 12-Dec-2019 Revision Date 30-Jul-2020 Revision Number 2 1. Identification Product identifier Product Name Ammonium Sulfamate Other means of identification Product Code(s) PN000173, PN002246 Recommended use of the chemical and restrictions on use Recommended use Laboratory reagent For professional use only Restrictions on use No information available. Details of the supplier of the safety data sheet Supplier Address Associates of Cape Cod, Inc. 124 Bernard E. Saint Jean Drive East Falmouth, MA 02536-4445 508 540 3444 E-mail [email protected] Emergency telephone number Emergency Telephone CHEMTREC: +1-703-527-3887 (INTERNATIONAL) 1-800-424-9300 (NORTH AMERICA) 2. Hazard(s) identification Classification Not classified. Label elements Hazard statements Not classified. Other information No information available. _____________________________________________________________________________________________ (M)SDS Number UL-ACC-015 R_REG_SDS_0189 Page 1 / 8 Ammonium Sulfamate Revision Date: 30-Jul-2020 _____________________________________________________________________________________________ 3. Composition/information on ingredients Substance Not applicable. Mixture Chemical name CAS No Weight-% Hazardous Material Date HMIRA filed and Information Review date exemption Act registry number granted (if (HMIRA registry #) applicable) Ammonium sulfamate 7773-06-0 1-4 - - 4. First-aid measures Description of first aid measures Inhalation Remove to fresh air. Eye contact Rinse thoroughly with plenty of water, also under the eyelids. Get medical attention if symptoms occur. Skin contact Wash skin with soap and water. -

United States Patent (19) 11) Patent Number: 4,634,789 Teller Et Al
United States Patent (19) 11) Patent Number: 4,634,789 Teller et al. 45 Date of Patent: "Jan. 6, 1987 54 CONVERSION OF ACETONTRILE TO 56) References Cited GLYCOLONTRILE AND/OR U.S. PATENT DOCUMENTS GLYCOLAMDE 3,516,789 6/1970 Sennewald et al. ......... 260/465.3 X (75) Inventors: Raymond G. Teller, Aurora; James F. 4,515,732 5/1985 Brazdil et al. .................... 260/465.6 Brazdil, Mayfield Village; Linda C. Glaeser, Middleburg Heights, all of OTHER PUBLICATIONS Ohio Deutsch et al.; J. Prabst. Chemie, 321 (1), (1979), pp. (73) Assignee: The Standard Oil Company, 137-40, Cleveland, Ohio Primary Examiner-Joseph Paul Brust * Notice: The portion of the term of this patent Attorney, Agent, or Firm-Charles S. Lynch; John E. subsequent to May 7, 2002 has been Miller; Larry W. Evans disclaimed. 21) Appl. No.: 643,207 57 ABSTRACT 22 Filed: Aug. 22, 1984 The vapor phase oxidation of acetonitrile with molecu lar oxygen in the presence or absence of water vapor to 51) Int. Cl. .................. C07C 121/34; CO7C 121/36; produce glycolonitrile or glycolamide in the presence CO7C 102/08 of a vanadium oxide based catalyst. 52 U.S. Cl. ..................................... 558/451; 558/457 58 Field of Search ..................... 260/465.6; 564/130; 558/451 10 Claims, No Drawings 4,634,789 1. 2 port being 5-95, usually 10-90 weight percent of the CONVERSION OF ACETONTRLE TO catalyst composition. GLYCOLONTRILE AND/OR GLYCOLAM DE Especially useful catalysts are where A is K, a is 0.005-0.05, b is zero and c is 2; and where B is Fe, a is This invention relates to a novel catalytic method of 5 zero, b is 0.05-0.5 and c is 2. -

Chapter 5206 Department of Labor and Industry Employee Right-To-Know Standards
MINNESOTA RULES 1985 4485 EMPLOYEE RIGHT-TO-KNOW STANDARDS 5206.0100 CHAPTER 5206 DEPARTMENT OF LABOR AND INDUSTRY EMPLOYEE RIGHT-TO-KNOW STANDARDS 5206.0100 DEFINITIONS. 5206.0900 CRITERIA FOR TECHNICALLY 5206.0200 PURPOSE. QUALIFIED INDIVIDUALS. 5206.0300 SCOPE; EXCEPTIONS. LABELING 5206.0400 HAZARDOUS SUBSTANCES. 5206.1000 LABELING HAZARDOUS SUBSTANCES. 5206.0500 HARMFUL PHYSICAL AGENTS. 5206.0600 INFECTIOUS AGENTS: HOSPITALS 5206.1100 LABELING HARMFUL PHYSICAL AND CLINICS. AGENTS: LABEL CONTENT. 5206 0700 TRAINING 5206 1200 CERTIFICATION OF EXISTING 5206.0800 AVAILABILITY OF INFORMATION. LABELING PROGRAM. 5206.0100 DEFINITIONS. Subpart 1. Scope. For purposes of this chapter the following terms have the meanings given them. Subp. 2. Commissioner. "Commissioner" means the commissioner of the Department of Labor and Industry. Subp. 3. Data sheet. "Data sheet" means a document, such as a material safety data sheet, operation standard, placard or display device, used by an employer to communicate to an employee the information required under Minnesota Statutes, section 182.653, subdivisions 4b, 4c, and 4e. Subp. 4. Department. "Department" means the Department of Labor and Industry. Subp. 5. Display device. "Display device" means a video screen or video display terminal that is part of electronic data processing equipment. Subp. 6. Harmful physical agent. "Harmful physical agent" means a physical agent determined by the commissioner as part of the standard for that agent to present a significant risk to worker health or safety or imminent danger of death or serious physical harm to an employee. "Harmful physical agent" does not include an agent being developed or utilized by a technically qualified individual in a research, medical research, medical diagnostic, or medical educational laboratory, or in a health care facility or in a clinic associated with the laboratory or health care facility, or in a pharmacy registered and licensed under Minnesota Statutes, chapter 151.