Organic Photovoltaic Module with High Total-Area Efficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MEMS Technology for Physiologically Integrated Devices
A BioMEMS Review: MEMS Technology for Physiologically Integrated Devices AMY C. RICHARDS GRAYSON, REBECCA S. SHAWGO, AUDREY M. JOHNSON, NOLAN T. FLYNN, YAWEN LI, MICHAEL J. CIMA, AND ROBERT LANGER Invited Paper MEMS devices are manufactured using similar microfabrica- I. INTRODUCTION tion techniques as those used to create integrated circuits. They often, however, have moving components that allow physical Microelectromechanical systems (MEMS) devices are or analytical functions to be performed by the device. Although manufactured using similar microfabrication techniques as MEMS can be aseptically fabricated and hermetically sealed, those used to create integrated circuits. They often have biocompatibility of the component materials is a key issue for moving components that allow a physical or analytical MEMS used in vivo. Interest in MEMS for biological applications function to be performed by the device in addition to (BioMEMS) is growing rapidly, with opportunities in areas such as biosensors, pacemakers, immunoisolation capsules, and drug their electrical functions. Microfabrication of silicon-based delivery. The key to many of these applications lies in the lever- structures is usually achieved by repeating sequences of aging of features unique to MEMS (for example, analyte sensitivity, photolithography, etching, and deposition steps in order to electrical responsiveness, temporal control, and feature sizes produce the desired configuration of features, such as traces similar to cells and organelles) for maximum impact. In this paper, (thin metal wires), vias (interlayer connections), reservoirs, we focus on how the biological integration of MEMS and other valves, or membranes, in a layer-by-layer fashion. The implantable devices can be improved through the application of microfabrication technology and concepts. -

Design of a Microelectronic Manufacturing Laboratory
2006-1635: DESIGN OF A MICROELECTRONIC MANUFACTURING LABORATORY Stilson Applin, Montana State University Todd Kaiser, Montana State University Page 11.407.1 Page © American Society for Engineering Education, 2006 Design of a Microelectronic Manufacturing Laboratory Abstract The design of an undergraduate microelectronic manufacturing laboratory for teaching will be described in the following paper. This laboratory emphasizes learning the processes of semiconductor manufacturing and clean room protocol. The laboratory is housed in a 500 square foot, class 10,000 facility. In the laboratory the students, with a junior standing and a science based background, will use a pre-made six mask set to create P and N type transistors as well as inverters and diodes. The students will be conducting oxidization, RCA clean, photolithography, etching, diffusion, metallization and other processes. A brief description of these processes and the methods used to teach them will also be described. In addition to these processes students will also learn about clean room protocol, chemical safety, and testing devices. All of these skills will be marketable to future employers and graduate schools. These same skills and processes will be covered in a seminar course for educators, with the main purpose of inspiring the high school teachers to teach about semiconductor manufacturing. The cost effective design is what makes the laboratory unique. The expenditure control is important due to the size of the Electrical Engineering department. The department has only 250 undergraduates and 40 graduate students, thus internal funding is difficult to obtain. A user fee paid by the students will cover the funding. This fee will be small and manageable for any college student. -

Advanced Solar Technology Sample
ADVANCED SOLAR TECHNOLOGY SAMPLE TECHNOLOGY ACQUISITION REPORT CONTENTS Technology 1: Microsystems Enabled Photovoltaics (MEPV) - Photovoltaic solar concentrator ............... 4 Technology 2: Method and apparatus for integrating an infrared (ir) photovoltaic cell on a thin film photovoltaic cell ............................................................................................................................................ 7 Technology 3: Photovoltaic cells with quantum dots with built-in-charge and methods of making same 10 Technology 4: Super-transparent electodes for photovoltaic applications ................................................. 13 Technology 5: Optical concentrator and associated photovoltaic devices ................................................. 15 Technology 6: Hybrid photovoltaic devices and applications thereof ........................................................ 18 Technology 7: Methods of manufacturing photovoltaic electrodes ............................................................ 20 Technology 8: Thin film photovoltaic cell structure, nanoantenna, and method for manufacturing ......... 23 Technology 9: Apparatuses, systems and methods for cleaning photovoltaic devices .............................. 26 Technology 10: Photovoltaic structures having a light scattering Interface layer and methods of making the same ....................................................................................................................................................... 28 Technology 11: Rapid thermal -

A Review of Current Methods in Microfluidic Device Fabrication And
inventions Review A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects Bruce K. Gale * ID , Alexander R. Jafek ID , Christopher J. Lambert ID , Brady L. Goenner, Hossein Moghimifam, Ugochukwu C. Nze ID and Suraj Kumar Kamarapu ID Department of Mechanical Engineering, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (A.R.J.); [email protected] (C.J.L.); [email protected] (B.L.G.); [email protected] (H.M.); [email protected] (U.C.N.); [email protected] (S.K.K.) * Correspondence: [email protected]; Tel.: +1-801-585-5944 Received: 30 June 2018; Accepted: 20 August 2018; Published: 28 August 2018 Abstract: Microfluidic devices currently play an important role in many biological, chemical, and engineering applications, and there are many ways to fabricate the necessary channel and feature dimensions. In this review, we provide an overview of microfabrication techniques that are relevant to both research and commercial use. A special emphasis on both the most practical and the recently developed methods for microfluidic device fabrication is applied, and it leads us to specifically address laminate, molding, 3D printing, and high resolution nanofabrication techniques. The methods are compared for their relative costs and benefits, with special attention paid to the commercialization prospects of the various technologies. Keywords: microfabrication; nanofabrication; microfluidics; nanofluidics; 3D printing; laminates; molding 1. Introduction Microfluidics is a growing field of research which pertains to the manipulation of fluids on the microscale level, and it is identified most commonly by devices with critical dimensions of less than 1 mm. -

Microfabrication and Microfluidics for 3D Brain-On-Chip
Microfabrication and microfluidics for 3D brain-on-chip Bart Schurink Microfabrication and microfluidics for 3D brain-on-chip Bart Schurink i Members of the committee: Chairman Prof. dr. ir. J.M.W. Hilgenkamp (voorzitter) Universiteit Twente Promotor Prof. dr. J.G.E. Gardeniers Universiteit Twente Co-promotor/supervisor Dr. R. Luttge Universiteit Eindhoven Members Prof. dr. J.C.T. Eijkel University of Twente Prof. dr. H.B.J. Karperien University of Twente Prof. dr. A.S. Holmes Imperial College London Prof. dr. ir. J.M.J. den Toonder Technical University of Eindhoven Prof. dr. ir. R. Dekker Delft University of Technology The work in this thesis was carried out in the Mesoscale Chemical Systems group and MESA+ Institute for Nanotechnology, University of Twente. It is part of the MESOTAS project financially supported by ERC, Starting Grant no. 280281 Title: Microfabrication and microfluidics for 3D brain-on-chip Author: Bart Schurink ISBN: 978-90-365-4142-8 DOI: 10.3990/1.9789036541428 Publisher: Gildeprint, Enschede, The Netherlands Copyright © 2016 by Bart Schurink, Enschede, The Netherlands. No part of the this book may be copied, photocopied, reproduced, translated or reduced to any electronic medium or machine-readable form, in whole or in part, without specific permission of the author. ii Microfabrication and microfluidics for 3D brain-on-chip PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit Twente, op gezag van de rector magnificus, Prof. dr. H. Brinksma, volgens besluit van het College voor Promoties in het openbaar te verdedigen op donderdag 23 juni 2016 door Bart Schurink Geboren op 6 november 1982 te Oldenzaal, Nederland iii Dit proefschrift is goedgekeurd door de promotor: Prof. -

A Technology Overview and Applications of Bio-MEMS
INSTITUTE OF SMART STRUCTURES AND SYSTEMS (ISSS) JOURNAL OF ISSS J. ISSS Vol. 3 No. 2, pp. 39-59, Sept. 2014. REVIEW ARTICLE A Technology Overview and Applications of Bio-MEMS Nidhi Maheshwari+, Gaurav Chatterjee+, V. Ramgopal Rao. Department of Electrical Engineering, Indian Institute of Technology Bombay, Mumbai, India-400076. Corresponding Author: [email protected] + Both the authors have contributed equally. Keywords: Abstract Bio-MEMS, immobilization, Miniaturization of conventional technologies has long cantilever, micro-fabrication, been understood to have many benefits, like: lower cost of biosensor. production, lower form factor leading to portable applications, and lower power consumption. Micro/Nano fabrication has seen tremendous research and commercial activity in the past few decades buoyed by the silicon revolution. As an offset of the same fabrication platform, the Micro-electro-mechanical- systems (MEMS) technology was conceived to fabricate complex mechanical structures on a micro level. MEMS technology has generated considerable research interest recently, and has even led to some commercially successful applications. Almost every smart phone is now equipped with a MEMS accelerometer-gyroscope system. MEMS technology is now being used for realizing devices having biomedical applications. Such devices can be placed under a subset of MEMS called the Bio-MEMS (Biological MEMS). In this paper, a brief introduction to the Bio-MEMS technology and the current state of art applications is discussed. 1. Introduction Generally, the Bio-MEMS can be defined as any The interdisciplinary nature of the Bio-MEMS research is system or device, which is fabricated using the highlighted in Figure 2. This highlights the overlapping of micro-nano fabrication technology, and used many different scientific disciplines, and the need for a healthy for biomedical applications such as diagnostics, collaborative effort. -

Design and Simulation of an RF-MEMS Switch and Analysis of Its
Advances in Science, Technology and Engineering Systems Journal Vol. 3, No. 1, 76-81 (2018) ASTESJ www.astesj.com ISSN: 2415-6698 Special issue on Advancement in Engineering Technology Design and Simulation of an RF-MEMS Switch and analysis of its Electromagnetic aspect in realtion to stress Amna Riaz*,1, Muhammad Umair Javed Ilam Sindhu2, Tahir Zaidi3 1University of Azad Jammu and Kashmir, Department of Electrical Engineering, Muzaffarabad, 13100, Pakistan 2Memorial University of Newfoundland, Department of Electrical and Computer Engineering, A1B 3X5, Canada 3National University of Science and Technology, Department of Electrical Engineering Department, EME College, 13100, Pakistan A R T I C L E I N F O A B S T R A C T Article history: Microelectromechanical Systems (MEMS) are devices made up of several electrical and Received: 31 May, 2017 mechanical components. They consist of mechanical functions (sensing, thermal, inertial) Accepted: 05 September, 2017 and electrical functions (switching, decision making) on a single chip made by Online: 30 January, 2018 microfabrication methods. These chips exhibit combined properties of the two functions. The size of system has characteristic dimensions less than 1mm but more than 1μm. The Keywords : configuration of these components determine the final deliverables of the switch. MEMS RF-MEMS can be designed to meet user requirements on any level from microbiological application Residual Stresses such as biomedical transducers or tissue engineering, to mechanical systems such as Coplanar waveguide microfluidic diagnoses or chemical fuel cells. The low cost, small mass and minimal power Skin depth consumption of the MEMS makes it possible to readily integrate to any kind of system in Return loss any environment. -

Microfabrication of Plastic-PDMS Microfluidic Devices Using Polyimide Release Layer and Selective Adhesive Bonding
BNL-114324-2017-JA Microfabrication of Plastic-PDMS Microfluidic Devices Using Polyimide Release Layer and Selective Adhesive Bonding S. Wang, M. Lu Submitted to the Journal of Micromechanics and Microengineering March 2017 Center for Functional Nanomaterials Brookhaven National Laboratory U.S. Department of Energy USDOE Office of Science (SC), Basic Energy Sciences (SC-22) Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No. DE- SC0012704 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. -

Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
micromachines Review Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems Jessica Ortigoza-Diaz 1, Kee Scholten 1 ID , Christopher Larson 1 ID , Angelica Cobo 1, Trevor Hudson 1, James Yoo 1 ID , Alex Baldwin 1 ID , Ahuva Weltman Hirschberg 1 and Ellis Meng 1,2,* 1 Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA; [email protected] (J.O.-D.); [email protected] (K.S.); [email protected] (C.L.); [email protected] (A.C.); [email protected] (T.H.); [email protected] (J.Y.); [email protected] (A.B.); [email protected] (A.W.H.) 2 Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA * Correspondence: [email protected]; Tel.: +1-213-740-6952 Received: 31 July 2018; Accepted: 18 August 2018; Published: 22 August 2018 Abstract: Parylene C is a promising material for constructing flexible, biocompatible and corrosion- resistant microelectromechanical systems (MEMS) devices. Historically, Parylene C has been employed as an encapsulation material for medical implants, such as stents and pacemakers, due to its strong barrier properties and biocompatibility. In the past few decades, the adaptation of planar microfabrication processes to thin film Parylene C has encouraged its use as an insulator, structural and substrate material for MEMS and other microelectronic devices. However, Parylene C presents unique challenges during microfabrication and during use with liquids, especially for flexible, thin film electronic devices. In particular, the flexibility and low thermal budget of Parylene C require modification of the fabrication techniques inherited from silicon MEMS, and poor adhesion at Parylene-Parylene and Parylene-metal interfaces causes device failure under prolonged use in wet environments. -

Creating a Solar Cell Array from a Single Silicon Wafer
Creating a Solar Cell Array from a Single Silicon Wafer Samyukta Gade – Materials Engineering Roderick Marstell – Materials Engineering Advisor: Dr. Richard Savage College of Engineering California Polytechnic State University San Luis Obispo 2012 Approval Page Project Title: Creating a Solar Cell Array from a Single Silicon Wafer Author: Samyukta Gade, Roderick Marstell Date Submitted: 6-1-2012 CAL POLY STATE UNIVERSITY Materials Engineering Department Since this project is a result of a class assignment, it has been graded and accepted as fulfillment of the course requirements. Acceptance does not imply technical accuracy or reliability. Any use of the information in this report, including numerical data, is done at the risk of the user. These risks may include catastrophic failure of the device or infringement of patent or copyright laws. The students, faculty, and staff of Cal Poly State University, San Luis Obispo cannot be held liable for any misuse of the project. Prof. Richard Savage ____________________________ Faculty Advisor Signature Prof. Trevor Harding ____________________________ Department Chair Signature ii Acknowledgments We would like to thank Dr. Savage for all the support he has given us during the design, planning, and execution. There is no way that we would have completed this project without his assistance. We also want to thank Elizabeth Brooks, Buddy Bump, and Nicolas Wiswell. They were instrumental in processing support and, in the case of Buddy, made testing possible. iii Abstract Two different processes to create multi-celled solar panels from single wafers were made and implemented. To do this, the cells must not have a common ground, and both designs use different approaches to solve this problem. -

MEMS and MEMS Microfabrication
MEMS and MEMS Microfabrication MEMS and MEMS Microfabrication What are MEMS? Micro Electro-Mechanical Systems (MEMS) are a technology field that can be defined as microscopic implementations of mechanical and electro-mechanical elements (ie devices and structures) that are made using the techniques of Microfabrication. These devices are widely used in everyday products such as Smartphones (microphones, gyroscope, magnetometer), Wearable devices (accelerometers, heart rate sensors), Automotive (pressure, flow, acceleration sensors) and many other applications. MEMS are everywhere Smartphones and Tablets Wearable Devices Automotive What are the differences between MEMS and Semiconductors? MEMS and Semiconductors are completely different technologies. Semiconductor devices make use of the electronic properties of materials such as Silicon. These properties have meant semiconductors now are the basis of most advanced electronics. Semiconductors have progressed since the 1960’s to the sophisticated microprocessors, memory chips, and wireless components that enable our Laptops, Tablets, and Smartphones, and the Internet of Things. Synergistically combined with Semiconductors, MEMS are Lab-on-a-chip essential in allowing devices to sense and physically interact with the world. MEMS devices can vary from a relatively simple structure to extremely complex electromechanical systems with multiple moving elements under the control of integrated microelectronics. MEMS can capture physical data such as measuring temperature, air pressure, magnetic fields and radiation. MEMS may use semiconductor material for its electronic qualities, but also utilize its mechanical properties. Silicon can be flexed with almost no energy dissipation, thereby providing highly repeatable motion and very little fatigue. Silicon based MEMS devices can have service lifetimes in the range trillions of cycles without failure. -
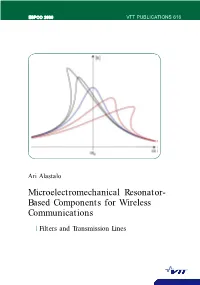
Microelectromechanical Resonator-Based Components for Wireless Communications
ESPOO 2006 VTT PUBLICATIONS 616 VTT PUBLICATIONS 616 PUBLICATIONS VTT Communications Wireless for Components ResonatorBased Microelectromechanical Starting in the early 1960's, when the integratedcircuit (IC) technology was developed, micromachining and microelectromechanical systems (MEMS) have grown into a broad research field with several commercial successes. Typical applications of MEMS are in physical, chemical and biochemical sensors, as well as in optical systems such as the digital micromirror device of Texas Instruments. From the 1990's, the advances in the processing technologies and the tremendous growth of the wirelesscommunication market have drawn much interest into radiofrequency MEMS devices (RF MEMS) such as filters, oscillators, switches and tunable capacitors. This thesis considers electrostaticallyactuated RFMEMS filters and delay lines. The intermodulation properties of capacitivelycoupled MEMS filters are analytically solved in closed form and the theory is verified in numerical simulations as well as in measurements with MEMS resonators. The theory is utilized to formulate a design procedure for MEMS filters that takes systems specifications for tolerable intermodulation distortion and insertionloss into account. For delay lines, capacitive actuation of bulk acoustic waves in a solid rod is analyzed. In particular, challenges in impedance matching due to the weakness of the electrostatic coupling are quantified. Finally, a new kind of resonatorchain delay line is introduced. This delay line is characterized by record•low acoustic signal velocity, narrowband response, and much lower characteristic impedance than Ari Alastalo found for the solid•rod waveguide enabling efficient signal coupling. Microelectromechanical Resonator Based Components for Wireless Communications Filters and Transmission Lines Tätä julkaisua myy Denna publikation säljs av This publication is available from VTT VTT VTT PL 1000 PB 1000 P.O.