Nasal Airflow Simulations Suggest Convergent Adaptation in Neanderthals and Modern Humans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Course Business Origin of Modern Humans
5/7/2014 Course Business • Today is the last lecture, an emotional day for us all • Final exam, next week; 6:30-9:00, same room Lecture Lab Text Book Australopiths Australopith Ch. 11 Diet Early Homo & Oldowan Early Homo Ch. 12 H. erectus: anatomy & behavior; Out of Africa 1 Homo erectus Ch. 13 Archaic H. sapiens: anatomy & behavior Late Homo Ch. 14 Neanderthal: anatomy & behavior Ch. 15 Modern H. sapiens: anatomy & behavior; Out of Africa 2 1 Origin of modern humans • Anatomically modern Homo sapiens • Behaviorally modern Homo sapiens • When, where, why? 2 1 5/7/2014 Hominin Date Ranges • Early hominins: 7.0 – 4.4 Ma • Gracile australopiths: 4.2 – 2.0 Ma • Robust australopiths: 2.3 – 1.2 Ma • Early Homo (H. habilis and H. rudolphensis): 2.0 – 1.6 Ma • Homo erectus: 1.8 mya – 50 ka • Archaic Homo sapiens (H. heidelbergensis): 600 – 125 ka • Homo neanderthalensis: 150 – 30 ka • Homo floresiensis: 90-11 ka • Anatomically modern Homo sapiens (AMHs): 195 ka 3 Modern human It’s complete!! Homo neanderthalensis Homo floresiensis Archaic Homo sapiens Paranthropus boisei Paranthropus robustus Homo erectus Homo rudolfensis Homo habilis Paranthropus aethiopicus Australopithecus africanus Australopithecus afarensis Australopithecus anamensis Ardipithecus (?) 4 2 5/7/2014 Cranial features of Homo sapiens: • Gracile skull (and postcranial anatomy) • Limited brow ridges, no superstructures • Rounded cranium with maximum breadth high on the vault • Orthognathic face • Small teeth and jaws • Obvious chin 5 Figure 14.01 6 3 5/7/2014 Diepkloof Rock Shelter -

An Anthropological Assessment of Neanderthal Behavioural Energetics
DEPARTMENT OF ARCHAEOLOGY, CLASSICS & EGYPTOLOGY An Anthropological Assessment of Neanderthal Behavioural Energetics. Thesis submitted in accordance with the requirements of the University of Liverpool for the Degree of Doctor in Philosophy by Andrew Shuttleworth. April, 2013. TABLE OF CONTENTS……………………………………………………………………..i LIST OF TABLES……………………………………………………………………………v LIST OF FIGURES…………………………………………………………………………..vi ACKNOWLEDGMENTS…………………………………………………………………...vii ABSTRACT…………………………………………………………………………………viii TABLE OF CONTENTS 1. INTRODUCTION...........................................................................................................1 1.1. Introduction..............................................................................................................1 1.2. Aims and Objectives................................................................................................2 1.3. Thesis Format...........................................................................................................3 2. THE NEANDERTHAL AND OXYEGN ISOTOPE STAGE-3.................................6 2.1. Discovery, Geographic Range & Origins..............................................................7 2.1.1. Discovery........................................................................................................7 2.1.2. Neanderthal Chronology................................................................................10 2.2. Morphology.............................................................................................................11 -

What's in a Neanderthal
WHAT’S IN A NEANDERTHAL: A COMPARATIVE ANALYSIS Taylorlyn Stephan Oberlin College Dept. of Anthropology Advised by Prof. Amy Margaris TABLE OF CONTENTS I. Abstract – pg. 3 II. Introduction – pg. 3-4 III. Historical Background – pg. 4-5 a. Fig. 1 – pg. 5 IV. Methods – pg. 5-8 a. Figs. 2 and 3 – pg. 6 V. Genomic Definitions – pg. 8-9 VI. Site Introduction – pg. 9-10 a. Fig 4 – pg. 10 VII. El Sidron – pg. 10-14 a. Table – pg. 10-12 b. Figs. 5-7 – pg. 12 c. Figs. 8 and 9 – pg. 13 VIII. Mezmaiskaya – pg. 14-18 a. Table – pg. 14-16 b. Figs. 10 and 11 – pg. 16 IX. Shanidar – pg. 18-22 a. Table – pg. 19-20 b. Figs. 12 and 13 – pg.21 X. Vindija – pg. 22-28 a. Table – pg. 23-25 b. Fig. 14 – pg. 25 c. Figs. 15-18 – pg. 26 XI. The Neanderthal Genome Project – pg. 28-32 a. Table – pg. 29 b. Fig. 19 – pg. 29 c. Figs. 20 and 21 – pg. 30 XII. Discussion – pg. 32- 36 XIII. Conclusion – pg. 36-38 XIV. Bibliography – pg. 38-42 2 ABSTRACT In this analysis, I seek to understand how three separate lines of evidence – skeletal morphology, archaeology, and genomics – are used separately and in tandem to produce taxonomic classifications in Neanderthal and paleoanthropological research more generally. To do so, I have selected four sites as case studies: El Sidrón Cave, Mezmaiskaya Cave, Shanidar Cave, and Vindija Cave. El Sidrón, Mezmaiskaya, and Vindija all have detailed archaeological records and have yielded Neanderthal DNA. -

Human Origin Sites and the World Heritage Convention in Eurasia
World Heritage papers41 HEADWORLD HERITAGES 4 Human Origin Sites and the World Heritage Convention in Eurasia VOLUME I In support of UNESCO’s 70th Anniversary Celebrations United Nations [ Cultural Organization Human Origin Sites and the World Heritage Convention in Eurasia Nuria Sanz, Editor General Coordinator of HEADS Programme on Human Evolution HEADS 4 VOLUME I Published in 2015 by the United Nations Educational, Scientific and Cultural Organization, 7, place de Fontenoy, 75352 Paris 07 SP, France and the UNESCO Office in Mexico, Presidente Masaryk 526, Polanco, Miguel Hidalgo, 11550 Ciudad de Mexico, D.F., Mexico. © UNESCO 2015 ISBN 978-92-3-100107-9 This publication is available in Open Access under the Attribution-ShareAlike 3.0 IGO (CC-BY-SA 3.0 IGO) license (http://creativecommons.org/licenses/by-sa/3.0/igo/). By using the content of this publication, the users accept to be bound by the terms of use of the UNESCO Open Access Repository (http://www.unesco.org/open-access/terms-use-ccbysa-en). The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of UNESCO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The ideas and opinions expressed in this publication are those of the authors; they are not necessarily those of UNESCO and do not commit the Organization. Cover Photos: Top: Hohle Fels excavation. © Harry Vetter bottom (from left to right): Petroglyphs from Sikachi-Alyan rock art site. -

2010 Conference
1 2 Thursday 8th April Chair: Name 10.00-10.20 Ian Candy Sedimentology and Palaeoenvironments of the Wroxham Formation: The geological context of the earliest humans in Britain 10.20-10.40 Simon Parfitt, Simon Lewis Where the wild things are. New evidence for early & Nick Ashton humans from East Anglia and the North Sea 10.40-11.00 Simon Lewis et al. The Ancestral River Thames in Norfolk: palaeogeography and human presence 11.00-11.10 Questions Chair: Name 11.10-11.40 Coffee 11.40-12.00 Justin Dix and Fraser Sturt Submerged Early Middle Pleistocene Palaeo- landscapes of the Thames Estuary 12.00-12.20 Mike Field Recent work at Happisburgh Site 1 12.20-12.40 Richard Preece & Simon The age of the Middle Pleistocene succession in Parfitt Norfolk and its relevance for Palaeolithic archaeology 12.40-1.00 Kirsty Penkman Dating the early Palaeolithic: the new aminostratigraphy 1.00-1.10 Questions Chair: Name 1.10-2.10 Lunch 2.10-2.30 Andreu Ollé et al. Experimental knapping and butchery: replicating Boxgrove 2.30-2.50 Laura Basell Tony Brown, A mixed assemblage and fluvio-periglacial Phil Toms, Chris Norman & sedimentation at Doniford, North Somerset Rob Hosfield 2.50-3.10 Rob Hosfield & Nick Mapping the human record in the British early Ashton Palaeolithic: evidence from the Solent River system 3.10-3.20 Questions 3.20-3.50 Tea Chair: Name 3.50-4.10 Kathy MacDonald Environmental tolerances of the earliest occupants of Europe: a review of the Leiden workshop and implications for future research 4.10-4.30 Matt Pope Human tool using behaviour and -

The Talking Neanderthals: What Do Fossils, Genetics and Archeology Say? Biolinguistics, 7, 35-74
Institutional repository of Jönköping University http://www.publ.hj.se/diva This is the publisher version of a paper published in Biolinguistics. This paper has been peer-reviewed. Citation for the published paper: Johansson, S. (2013) The Talking Neanderthals: What do Fossils, Genetics and Archeology Say? Biolinguistics, 7, 35-74 Publishers homepage: http://www.biolinguistics.eu/index.php/biolinguistics/index Published with permission from: Biolinguistics The Talking Neanderthals: What Do Fossils, Genetics, and Archeology Say? Sverker Johansson Did Neanderthals have language? This issue has been debated back and forth for decades, without resolution. But in recent years new evidence has become available. New fossils and archeological finds cast light on relevant Neanderthal anatomy and behavior. New DNA evidence, both fossil and modern, provides clues both to the relationship between Neanderthals and modern humans, and to the genetics of language. In this paper, I review and evaluate the available evidence. My conclusion is that the preponderance of the evidence supports the presence of at least a spoken proto-language with lexical semantics in Neanderthals. Keywords: archeology; DNA; fossils; language; Neanderthal 1. Introduction That modern humans have language and speech, and that our remote ancestors did not, are two incontrovertible facts. But there is no consensus on when the transition from non-language to language took place, nor any consensus on the species of the first language users. Some authors regard language as the exclusive province of anatomically modern humans [AMH] (Klein 1999, Skoyles & Sagan 2002, Crow 2005, Lanyon 2006, among others), whereas others argue that at least proto-language in some form, if not full modern language, can be found in some earlier species (Mithen 2005, Bickerton 2009, Corballis 2002, among others). -

A Comprehensive Analysis of Long Bone Curvature in Neanderthals and Modern Humans Using 3D Morphometries
A Comprehensive Analysis of Long Bone Curvature in Neanderthals and Modern Humans Using 3D morphometries Isabelle Elisabeth Peter Maria De Groote A thesis submitted to University College London in fulfillment of the requirements for the degree of Doctor of Philosophy. 2008 UMI Number: U592544 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U592544 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 I, Isabelle De Groote, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. ii Abstract Since their discovery Neanderthals were described as having a marked degree of anteroposterior curvature of the femoral shaft. Although initially believed to be pathological, subsequent discoveries of Neanderthal remains made femoral curvature as well as the lateral curvature of the radius to be considered derived Neanderthal features. Femoral curvature has previously been used in racial identification in modern humans but its functional significance is poorly understood. A recent study on Neanderthals and early modern humans found no differences in femoral curvature, but did not consider size-corrected curvature. -
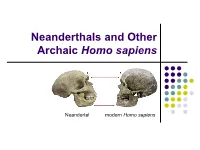
Chapter 10 Neanderthals and Archaic Homo Sapiens
Neanderthals and Other Archaic Homo sapiens Neandertal modern Homo sapiens Homo erectus → Modern Humans The evolutionary dividing line between Homo erectus and modern humans was not sharp, but extended over several hundred thousand years. Some regions were ahead of others in the process of evolving into our species. The evolutionary changes may have begun in Southern Europe and Northwest Africa by at least 600,000 years ago. Elsewhere in the Old World, this change apparently began around 400,000 years ago. Early Archaic Homo sapiens The transition to Homo sapiens was not complete until around 100,000 years ago and even somewhat later in some regions. Early archaic forms show morphological changes compared with H. erectus: brain expansion increased parietal breadth some decrease in the size of the molars general decrease in cranial and postcranial robusticity Early Archaic Homo sapiens Conservative taxonomy classifies all specimens as archaic forms within the species Homo sapiens. Other paleoanthropologists classify most specimens into other species of the genus Homo. In this view, some earlier archaic forms could be ancestral to modern humans. The Timeline By 100,000 BP some of the later archaic Homo sapiens had evolved into modern Homo sapiens, and in at least one area of Southeast Asia, Homo erectus remained until around 60,000 years ago. Archaic Homo sapiens Discoveries (Outside of Europe) Site Dates (ya) Human Remains Africa Bodo Middle Pleistocene Incomplete skull, part (Ethiopia) (600,000?) of braincase. Broken Hill -

The Gibraltar Museum Open Day 2018
PRESS RELEASE No: 269/2018 Date: 15th May 2018 The Gibraltar Museum Open Day 2018 The Gibraltar Museum will be opening its doors for their 17th annual Open Day on Saturday 19th May 2018 from 10:00 to 18:00, which as in other years is aimed to coincide as closely as possible with International Museum Day (18 May). This year’s international theme is “Hyperconnected museums: New approaches, new publics”. International Museum Day has been celebrated in Gibraltar since 2002 in the form of an open day which continues to increase in popularity within the local community. The Open Day promises to be fun-filled with plenty of activities, primarily aimed at young people, but also for the young at heart. The staff at the Gibraltar Museum will be on hand to answer any questions and, as in previous years, there will be a range of hands-on activities for all to enjoy. The Gibraltar Museum will also count on contributions from the Gibraltar National Archives and the Nautilus Project. The popular children’s events, such as the treasure hunt and fancy dress competition will once again feature on the day, with prizes for the winners. This year’s fancy dress competition will carry an open historical theme. Participants are asked to register on the day from 10:00 to 12:30. Come and meet Martin the Neanderthal This year’s event will also include a special exhibition of an articulated Neanderthal skeleton reproduction which will be on display to the Gibraltarian public for the first time. In the absence of a complete Neanderthal skeleton anywhere, this reproduction is the next best currently available. -

Neanderthals
Evo Edu Outreach (2010) 3:367–376 DOI 10.1007/s12052-010-0250-0 ORIGINAL SCIENTIFIC ARTICLE Neanderthals Katerina Harvati Published online: 23 July 2010 # Springer Science+Business Media, LLC 2010 Abstract Neanderthals are a group of fossil humans that The Discovery of Neanderthals inhabited Western Eurasia from approximately 300 to 30,000 years ago (ka). They vanished from the fossil record Neanderthal remains were already discovered in the early a few millennia after the first modern humans appeared in nineteenth century (Engis child in 1830, Forbes Quarry Europe (ca. 40 ka BP). They are characterized by a unique adult in 1848), but their significance was not recognized combination of distinctive anatomical features, and are until the discovery of the skeleton from the Neander valley found with stone tools of the Mousterian stone tool in 1856, roughly coinciding with the publication of industry. Current consensus views them as a distinct Darwin’s The Origin of Species in 1859. This locality Eurasian human lineage isolated from the rest of the Old lends its name to the group, and this discovery marks the World and sharing a common ancestor with modern beginning of the continuing debate surrounding the rela- humans sometime in the early Middle Pleistocene. The tionship of Neanderthals with modern humans. extreme cold of the European Ice Ages is considered at The antiquity of the Neanderthal skeleton and its status least partly responsible for the evolution of some of the as an extinct kind of human were not immediately accepted. distinctive Neanderthal anatomy, although other factors Instead, its peculiar anatomy was attributed to various (functional demands, effects of chance in small popula- pathologies, including rickets. -

Great Leap Forward
aAntng puv uortn~on~~ayJ A hardcover edition of this book was published in 1992 by HarperCollins PublLhers. THE TEWD CHIMPANZEX. Copyright O IW by Jared Diamond. All rights reserved. Printed in the United States of America. No part of this book may be used or reproduced in any manner whauo&e; without written permission except in the case of brief cpota&ns embodied in critical articles and reviews. For information address HarperCo1h.s Publishers, Inc., 10 East nrd Street, New York, NY 10022. HarperCollins books may be purchased for educational, business, or sales promotional use. For information please write: Special Markets Department, HarperCollins Publishers, Inc., 10 East grd Street, New York, NY 10022. First Harperperennial edition published 1993. Designed by Ruth KoIbert The Library of Congress has catalogued the hardcover edition as follows: Diamopd, ~aredM. The third chimpaniee : the evolution and future of the human animal / Jared Diamond. - sted. p. cm. Includes bibliographical references and index. ISBN 0-06-018307-1(cloth) I. Human evolution. 2. Socia evolution. 3. Man-Influence on nature. I. Title. GN2&,Ds 199 . ' m.2---dc20 91-50455 ISBN o-06-098403-I (~bk.) CHAPTER 2 Tbe Great Leap Forward I ! , 'FOR MOST OF THE MANY MILLIONS OF YEARS SINCE OUR LINEAGE ? diverged from that of apes, we remained little more than glorified I chimpanzees in how we made our living. As recently as forty thou- i sand years ago, western Europe was still occupied by Neanderthals, I I primitive beings for whom art and progress scarcely existed. Then i there came an abrupt change, as anatomically modern people ap- ! peared in Europe,. -

The Pleasures and Pitfalls of Teaching Human Evolution in the Museum
Evo Edu Outreach (2010) 3:403–409 DOI 10.1007/s12052-010-0252-y ORIGINAL SCIENTIFIC ARTICLE The Pleasures and Pitfalls of Teaching Human Evolution in the Museum Monique Scott Published online: 21 August 2010 # Springer Science+Business Media, LLC 2010 Abstract As natural history museums are increasingly on a frigid night. One dogmatic proponent prefers to describe challenged to represent the complexities of human evolu- himself as “a pre-Promethean sort of caveman. Much of his tion, new innovations are required to create dynamic, nourishment comes from grass-fed beef, which he eats raw. dialogic experiences between museum scientists and muse- In a bow to the times, he sometimes uses a fork.” Not um audiences. After the opening of the Spitzer Hall of surprisingly, selected members of the tribe were photo- Human Origins in 2006, I had the opportunity to take my graphed in front of a diorama at the American Museum of academic experience in physical anthropology to public Natural History (AMNH), a diorama featuring reconstruc- education in the Sackler Educational Laboratory for tions of modern humans from the Ukraine at 40,000 years Comparative Genomics and Human Origins. In the lab, I ago in the Hall of Human Origins. was able to intervene in museum visitors’ preconceptions of Fortunately or unfortunately, we do not live in the human evolution in a novel, informal educational setting. Paleolithic Age. Rather, we live in an educationally challenged age where fanciful images of “cavemen” have Keywords Anthropology . Human evolution . Museum become canonized as commonsense, if these urban “prim- studies . Informal learning .