Material and Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Vascular Plants
Guidelines for the Selection of Biological SSSIs Part 2: Detailed Guidelines for Habitats and Species Groups Chapter 11 Vascular Plants Authors Ian Taylor, Simon J. Leach, John P. Martin, Robert A. Jones, Julian Woodman and Iain Macdonald To view other Part 2 chapters and Part 1 of the SSSI Selection Guidelines visit: https://jncc.gov.uk/our-work/guidelines-for-selection-of-sssis/ Cite as: Taylor, I., Leach, S. J., Martin, J. P., Jones, R. A., Woodman, J. and Macdonald, I. 2021. Guidelines for the Selection of Biological SSSIs. Part 2: Detailed Guidelines for Habitats and Species Groups. Chapter 11 Vascular Plants. Joint Nature Conservation Committee, Peterborough. © Joint Nature Conservation Committee 2021 Guidelines for the Selection of Biological SSSIs – Part 2: Chapter 11 Vascular Plants (2021 revision v1.0) Cover note This chapter updates and replaces the previous Vascular Plant (VP) SSSI selection guidelines for vascular plants (JNCC 1989). It was drafted initially by Ian Taylor, Simon J. Leach and John P. Martin (NE) and Robert A. Jones (NRW), with the final draft in November 2020 produced by Ian Taylor (NE), Julian Woodman (NRW) and Iain Macdonald (NatureScot). It provides detailed guidance for selecting vascular plant sites throughout Great Britain to recommend for notification as SSSIs. It should be used in conjunction with Part 1 of the SSSI Selection Guidelines (Bainbridge et al. 2013), which details the overarching rationale, operational approach and criteria for the selection of SSSIs. The main changes from the previous vascular plant guidelines are: • a change of emphasis in favour of a species-by-species focus versus an in- combination (or assemblage) focus. -

Honey and Pollen Flora of SE Australia Species
List of families - genus/species Page Acanthaceae ........................................................................................................................................................................34 Avicennia marina grey mangrove 34 Aizoaceae ............................................................................................................................................................................... 35 Mesembryanthemum crystallinum ice plant 35 Alliaceae ................................................................................................................................................................................... 36 Allium cepa onions 36 Amaranthaceae ..................................................................................................................................................................37 Ptilotus species foxtails 37 Anacardiaceae ................................................................................................................................................................... 38 Schinus molle var areira pepper tree 38 Schinus terebinthifolius Brazilian pepper tree 39 Apiaceae .................................................................................................................................................................................. 40 Daucus carota carrot 40 Foeniculum vulgare fennel 41 Araliaceae ................................................................................................................................................................................42 -

Centaurea Sect
Tesis Doctoral ESTUDIO TAXONÓMICO DE CENTAUREA SECT. SERIDIA (JUSS.) DC. (ASTERACEAE) EN LA PENÍNSULA IBÉRICA E ISLAS BALEARES Memoria presentada por Dña. Vanessa Rodríguez Invernón para optar al grado de Doctor en Ciencias Biológicas por la Universidad de Córdoba Director de Tesis: Prof. Juan Antonio Devesa 15 de octubre de 2013 TITULO: Estudio taxonómico de Centaurea Sect. Seridia (Juss.) DC. en la Península Ibérica e Islas Baleares AUTOR: Vanessa Rodríguez Invernón © Edita: Servicio de Publicaciones de la Universidad de Córdoba. 2013 Campus de Rabanales Ctra. Nacional IV, Km. 396 A 14071 Córdoba www.uco.es/publicaciones [email protected] rírulo DE LA TESIS: Estudio Taxonómico de centaurea sect. seridia (Juss.) DG. en la Península lbérica e Islas Baleares DOCTORANDO/A: VANESSA RODRíGUEZ INVERNÓN INFORME RAZONADO DEL/DE LOS DIRECTOR/ES DE LA TESIS (se hará mención a la evolución y desarrollo de la tesis, así como a trabajos y publicaciones derivados de la misma). El objeto de esta Tesis Doctoral ha sido el estudio taxonómico del género Centaurea, cuya diversidad y complejidad en el territorio es alta, por lo que se ha restringido a la sección Seridia (Juss.) DC. y, atin así, el estudio ha requerido 4 años de dedicación para su finalización. La iniciativa se inscribe en el Proyecto Flora iberica, financiado en la actualidad por el Ministerio de Economía y Competitividad. El estudio ha entrañado la realización de numerosas prospecciones en el campo, necesarias para poder abordar aspectos importantes, tales como los estudios cariológicos, palinológicos y moleculares, todos encaminados a apoyar la slntesis taxonómica, que ha requerido además de un exhaustivo estudio de material conservado en herbarios nacionales e internacionales. -

Gathered Food Plants in the Mountains
34063_Rivera.qxd 7/2/07 2:03 PM Page 1 Gathered Food Plants in the Mountains of Castilla–La Mancha (Spain): Ethnobotany and Multivariate Analysis1 Diego Rivera*,2, Concepción Obón3, Cristina Inocencio3, Michael Heinrich4, Alonso Verde2, José Fajardo2, and José Antonio Palazón5 2 Departamento de Biología Vegetal, Facultad de Biología, Universidad de Murcia, 30100 Murcia, Spain 3 Departamento de Biología Aplicada, EPSO, Universidad Miguel Hernández, 03312 Orihuela, Alicante, Spain 4 Centre for Pharmacognosy and Phytotherapy, The School of Pharmacy, Univ. London, 29–39 Brunswick Sq. London, WC1N 1AX, United Kingdom 5 Departamento de Ecología e Hidrología, Facultad de Biología, Universidad de Murcia, 30100 Murcia, Spain * Corresponding author: Departamento de Biología Vegetal, Facultad de Biología, Universidad de Murcia, 30100 Murcia, Spain; e-mail: [email protected] GATHERED FOOD PLANTS IN THE MOUNTAINS OF CASTILLA–LA MANCHA (SPAIN): ETHNOBOTANY AND MULTIVARIATE ANALYSIS. Gathered food plants (GFPs) (wild and weeds) are crucial for under- standing traditional Mediterranean diets. Combining open interviews and free–listing ques- tionnaires, we identified 215 GFP items, i.e., 53 fungi and 162 from 154 vascular plant species. The variation in frequency and in salience among the items follows a rectangular hyperbola. Highly salient species were Silene vulgaris (Moench) Garcke, Scolymus hispani- cus L., and Pleurotus eryngii (DC.: Fr.) Quélet. Salience and frequency showed no correlation with the expected health benefits of each species. Regional frequency in the Mediter- ranean and local frequency are directly related. Thus, local food plants are much less “local” than expected. Different types of culinary preparations provide the most information in the cluster analysis of variables. -

Evaluation of Drought Resistance and Transcriptome Analysis For
www.nature.com/scientificreports OPEN Evaluation of drought resistance and transcriptome analysis for the identifcation of drought‑responsive genes in Iris germanica Jingwei Zhang1, Dazhuang Huang1*, Xiaojie Zhao1 & Man Zhang2 Iris germanica, a species with very high ornamental value, exhibits the strongest drought resistance among the species in the genus Iris, but the molecular mechanism underlying its drought resistance has not been evaluated. To investigate the gene expression profle changes exhibited by high‑ drought‑resistant I. germanica under drought stress, 10 cultivars with excellent characteristics were included in pot experiments under drought stress conditions, and the changes in the chlorophyll (Chl) content, plasma membrane relative permeability (RP), and superoxide dismutase (SOD), malondialdehyde (MDA), free proline (Pro), and soluble protein (SP) levels in leaves were compared among these cultivars. Based on their drought‑resistance performance, the 10 cultivars were ordered as follows: ‘Little Dream’ > ‘Music Box’ > ‘X’Brassie’ > ‘Blood Stone’ > ‘Cherry Garden’ > ‘Memory of Harvest’ > ‘Immortality’ > ‘White and Gold’ > ‘Tantara’ > ‘Clarence’. Using the high‑drought‑resistant cultivar ‘Little Dream’ as the experimental material, cDNA libraries from leaves and rhizomes treated for 0, 6, 12, 24, and 48 h with 20% polyethylene glycol (PEG)‑6000 to simulate a drought environment were sequenced using the Illumina sequencing platform. We obtained 1, 976, 033 transcripts and 743, 982 unigenes (mean length of 716 bp) through a hierarchical clustering analysis of the resulting transcriptome data. The unigenes were compared against the Nr, Nt, Pfam, KOG/COG, Swiss‑Prot, KEGG, and gene ontology (GO) databases for functional annotation, and the gene expression levels in leaves and rhizomes were compared between the 20% PEG‑6000 stress treated (6, 12, 24, and 48 h) and control (0 h) groups using DESeq2. -

Inter-Regional Hybrids of Native and Non-Native Centaurea Sulphurea
Plant Ecology & Diversity ISSN: 1755-0874 (Print) 1755-1668 (Online) Journal homepage: http://www.tandfonline.com/loi/tped20 Inter-regional hybrids of native and non- native Centaurea sulphurea inherit increased competitive ability from the non-natives João C. Filipe & Daniel Montesinos To cite this article: João C. Filipe & Daniel Montesinos (2016) Inter-regional hybrids of native and non-native Centaurea sulphurea inherit increased competitive ability from the non- natives, Plant Ecology & Diversity, 9:4, 381-386, DOI: 10.1080/17550874.2016.1261950 To link to this article: http://dx.doi.org/10.1080/17550874.2016.1261950 Published online: 06 Dec 2016. Submit your article to this journal Article views: 18 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tped20 Download by: [Universidad de Sevilla] Date: 01 February 2017, At: 05:14 Plant Ecology & Diversity, 2016 Vol. 9, No. 4, 381–386, http://dx.doi.org/10.1080/17550874.2016.1261950 SHORT COMMUNICATION Inter-regional hybrids of native and non-native Centaurea sulphurea inherit increased competitive ability from the non-natives João C. Filipe* and Daniel Montesinos Centre for Functional Ecology, Department of Life Sciences, University of Coimbra, Coimbra, Portugal (Received 16 June 2016; accepted 14 November 2016) Background: Exotic species can rapidly develop adaptations to their non-native regions, such as increased size and competitive ability. Although these traits are believed to be responsible for invasive success, some non-invasive exotic species display them too. This suggests that increased size and competitive ability might be necessary but not sufficient to turn an exotic into a successful invader. -

Nuclear and Plastid DNA Phylogeny of the Tribe Cardueae (Compositae
1 Nuclear and plastid DNA phylogeny of the tribe Cardueae 2 (Compositae) with Hyb-Seq data: A new subtribal classification and a 3 temporal framework for the origin of the tribe and the subtribes 4 5 Sonia Herrando-Morairaa,*, Juan Antonio Callejab, Mercè Galbany-Casalsb, Núria Garcia-Jacasa, Jian- 6 Quan Liuc, Javier López-Alvaradob, Jordi López-Pujola, Jennifer R. Mandeld, Noemí Montes-Morenoa, 7 Cristina Roquetb,e, Llorenç Sáezb, Alexander Sennikovf, Alfonso Susannaa, Roser Vilatersanaa 8 9 a Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s.n., 08038 Barcelona, Spain 10 b Systematics and Evolution of Vascular Plants (UAB) – Associated Unit to CSIC, Departament de 11 Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de 12 Barcelona, ES-08193 Bellaterra, Spain 13 c Key Laboratory for Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, 14 Chengdu, China 15 d Department of Biological Sciences, University of Memphis, Memphis, TN 38152, USA 16 e Univ. Grenoble Alpes, Univ. Savoie Mont Blanc, CNRS, LECA (Laboratoire d’Ecologie Alpine), FR- 17 38000 Grenoble, France 18 f Botanical Museum, Finnish Museum of Natural History, PO Box 7, FI-00014 University of Helsinki, 19 Finland; and Herbarium, Komarov Botanical Institute of Russian Academy of Sciences, Prof. Popov str. 20 2, 197376 St. Petersburg, Russia 21 22 *Corresponding author at: Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s. n., ES- 23 08038 Barcelona, Spain. E-mail address: [email protected] (S. Herrando-Moraira). 24 25 Abstract 26 Classification of the tribe Cardueae in natural subtribes has always been a challenge due to the lack of 27 support of some critical branches in previous phylogenies based on traditional Sanger markers. -

A Probable Oligochaete from an Early Triassic Lagerstätte of the Southern Cis-Urals and Its Evolutionary Implications
Editors' choice A probable oligochaete from an Early Triassic Lagerstätte of the southern Cis-Urals and its evolutionary implications DMITRY E. SHCHERBAKOV, TARMO TIMM, ALEXANDER B. TZETLIN, OLEV VINN, and ANDREY Y. ZHURAVLEV Shcherbakov, D.E., Timm, T., Tzetlin, A.B., Vinn, O., and Zhuravlev, A.Y. 2020. A probable oligochaete from an Early Triassic Lagerstätte of the southern Cis-Urals and its evolutionary implications. Acta Palaeontologica Polonica 65 (2): 219–233. Oligochaetes, despite their important role in terrestrial ecosystems and a tremendous biomass, are extremely rare fossils. The palaeontological record of these worms is restricted to some cocoons, presumable trace fossils and a few body fossils the most convincing of which are discovered in Mesozoic and Cenozoic strata. The Olenekian (Lower Triassic) siliciclastic lacustrine Petropavlovka Lagerstätte of the southern Cis-Urals yields a number of extraordinary freshwater fossils including an annelid. The segmented body with a secondary annulation of this fossil, a subtriangular prostomium, a relatively thick layered body wall and, possibly, the presence of a genital region point to its oligochaete affinities. Other fossil worms which have been ascribed to clitellates are reviewed and, with a tentative exception of two Pennsylvanian finds, affinities of any pre-Mesozoic forms to clitellate annelids are rejected. The new fossil worm allows tracing of a persuasive oligochaete record to the lowermost Mesozoic and confirms a plausibility of the origin of this annelid group in freshwater conditions. Key words: Annelida, Clitellata, Oligochaeta, Mesozoic, Lagerstätte, Russia. Dmitry E. Shcherbakov [[email protected]], Borissiak Palaeontological Institute, Russian Academy of Sciences, Profso- yuz naya St 123, Moscow 117647, Russia. -

THE IRISH RED DATA BOOK 1 Vascular Plants
THE IRISH RED DATA BOOK 1 Vascular Plants T.G.F.Curtis & H.N. McGough Wildlife Service Ireland DUBLIN PUBLISHED BY THE STATIONERY OFFICE 1988 ISBN 0 7076 0032 4 This version of the Red Data Book was scanned from the original book. The original book is A5-format, with 168 pages. Some changes have been made as follows: NOMENCLATURE has been updated, with the name used in the 1988 edition in brackets. Irish Names and family names have also been added. STATUS: There have been three Flora Protection Orders (1980, 1987, 1999) to date. If a species is currently protected (i.e. 1999) this is stated as PROTECTED, if it was previously protected, the year(s) of the relevant orders are given. IUCN categories have been updated as follows: EN to CR, V to EN, R to V. The original (1988) rating is given in brackets thus: “CR (EN)”. This takes account of the fact that a rare plant is not necessarily threatened. The European IUCN rating was given in the original book, here it is changed to the UK IUCN category as given in the 2005 Red Data Book listing. MAPS and APPENDIX have not been reproduced here. ACKNOWLEDGEMENTS We are most grateful to the following for their help in the preparation of the Irish Red Data Book:- Christine Leon, CMC, Kew for writing the Preface to this Red Data Book and for helpful discussions on the European aspects of rare plant conservation; Edwin Wymer, who designed the cover and who, as part of his contract duties in the Wildlife Service, organised the computer applications to the data in an efficient and thorough manner. -

First Genome Size Assessments in 'Carduncellus'
Collectanea Botanica 40: e004 enero-diciembre 2021 ISSN-L: 0010-0730 https://doi.org/10.3989/collectbot.2021.v40.004 First genome size assessments in Carduncellus and its related genera Femeniasia and Phonus (Asteraceae, Cardueae), with data on 21 taxa TERESA GARNATJE1, ORIANE HIDALGO1, JOAN VALLÈS2, SÒNIA GARCIA1, ÀNGEL ROMO1 & ROSER VILATERSANA1 1 Institut Botànic de Barcelona (IBB, CSIC-Ajuntament de Barcelona), pg. del Migdia, s/n, Parc de Montjuïc, ES- 08038 Barcelona, Catalonia, Spain 2 Laboratori de Botànica - Unitat associada CSIC, Facultat de Farmàcia i Ciències de l’Alimentació - Institut de la Biodiversitat IRBio, Universitat de Barcelona, av. Joan XXIII, 27-31, 08028 Barcelona ORCID iD. T. GARNATJE: https://orcid.org/0000-0001-6295-6217, O. HIDALGO: https://orcid.org/0000-0002-1547-8627, J. VALLÈS: https://orcid.org/0000-0002-1309-3942, S. GARCIA: http://orcid.org/0000-0002-3143-0527, À. ROMO: https://orcid.org/0000-0001-8135-8570, R. VILATERSANA: https://orcid.org/0000-0002-5106-8764 Author for correspondence: T. Garnatje ([email protected]) Editor: Jordi López-Pujol Received 9 October 2020; accepted 26 January 2021; published on line xx Xxxx 2021 Abstract FIRST GENOME SIZE ASSESSMENTS IN CARDUNCELLUS AND ITS RELATED GENERA FEMENIASIA AND PHONUS (ASTERACEAE, CARDUEAE), WITH DATA ON 21 TAXA.— Genome size of 18 species of the genus Carduncellus, two species of the related genus Phonus and the monotypic genus Femeniasia (F. balearica) has been assessed by flow cytometry for the first time. Ploidy levels were assigned using genome size data together with previously reported chromosome counts. A phylogenetic framework was built to visualize how cytogenetic traits distributed across taxa. -
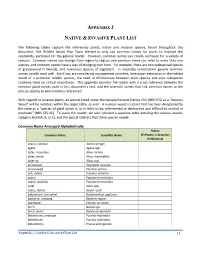
Reference Plant List
APPENDIX J NATIVE & INVASIVE PLANT LIST The following tables capture the referenced plants, native and invasive species, found throughout this document. The Wildlife Action Plan Team elected to only use common names for plants to improve the readability, particular for the general reader. However, common names can create confusion for a variety of reasons. Common names can change from region-to-region; one common name can refer to more than one species; and common names have a way of changing over time. For example, there are two widespread species of greasewood in Nevada, and numerous species of sagebrush. In everyday conversation generic common names usually work well. But if you are considering management activities, landscape restoration or the habitat needs of a particular wildlife species, the need to differentiate between plant species and even subspecies suddenly takes on critical importance. This appendix provides the reader with a cross reference between the common plant names used in this document’s text, and the scientific names that link common names to the precise species to which writers referenced. With regards to invasive plants, all species listed under the Nevada Revised Statute 555 (NRS 555) as a “Noxious Weed” will be notated, within the larger table, as such. A noxious weed is a plant that has been designated by the state as a “species of plant which is, or is likely to be, detrimental or destructive and difficult to control or eradicate” (NRS 555.05). To assist the reader, we also included a separate table detailing the noxious weeds, category level (A, B, or C), and the typical habitats that these species invade.