Descriptions of Amaricoccus Veronensis Sp. Nov., Amaricoccus Tamworthensis Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

Horizontal Operon Transfer, Plasmids, and the Evolution of Photosynthesis in Rhodobacteraceae
The ISME Journal (2018) 12:1994–2010 https://doi.org/10.1038/s41396-018-0150-9 ARTICLE Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae 1 2 3 4 1 Henner Brinkmann ● Markus Göker ● Michal Koblížek ● Irene Wagner-Döbler ● Jörn Petersen Received: 30 January 2018 / Revised: 23 April 2018 / Accepted: 26 April 2018 / Published online: 24 May 2018 © The Author(s) 2018. This article is published with open access Abstract The capacity for anoxygenic photosynthesis is scattered throughout the phylogeny of the Proteobacteria. Their photosynthesis genes are typically located in a so-called photosynthesis gene cluster (PGC). It is unclear (i) whether phototrophy is an ancestral trait that was frequently lost or (ii) whether it was acquired later by horizontal gene transfer. We investigated the evolution of phototrophy in 105 genome-sequenced Rhodobacteraceae and provide the first unequivocal evidence for the horizontal transfer of the PGC. The 33 concatenated core genes of the PGC formed a robust phylogenetic tree and the comparison with single-gene trees demonstrated the dominance of joint evolution. The PGC tree is, however, largely incongruent with the species tree and at least seven transfers of the PGC are required to reconcile both phylogenies. 1234567890();,: 1234567890();,: The origin of a derived branch containing the PGC of the model organism Rhodobacter capsulatus correlates with a diagnostic gene replacement of pufC by pufX. The PGC is located on plasmids in six of the analyzed genomes and its DnaA- like replication module was discovered at a conserved central position of the PGC. A scenario of plasmid-borne horizontal transfer of the PGC and its reintegration into the chromosome could explain the current distribution of phototrophy in Rhodobacteraceae. -

Key Role of Alphaproteobacteria and Cyanobacteria in the Formation of Stromatolites of Lake Dziani Dzaha (Mayotte, Western Indian Ocean)
fmicb-09-00796 May 18, 2018 Time: 17:53 # 1 ORIGINAL RESEARCH published: 22 May 2018 doi: 10.3389/fmicb.2018.00796 Key Role of Alphaproteobacteria and Cyanobacteria in the Formation of Stromatolites of Lake Dziani Dzaha (Mayotte, Western Indian Ocean) Emmanuelle Gérard1*, Siham De Goeyse1, Mylène Hugoni2, Hélène Agogué3, Laurent Richard4, Vincent Milesi1, François Guyot5, Léna Lecourt1, Stephan Borensztajn1, Marie-Béatrice Joseph1, Thomas Leclerc1, Gérard Sarazin1, Didier Jézéquel1, Christophe Leboulanger6 and Magali Ader1 1 UMR CNRS 7154 Institut de Physique du Globe de Paris, Sorbonne Paris Cité, Université Paris Diderot, Centre National de la Recherche Scientifique, Paris, France, 2 Université Lyon 1, UMR CNRS 5557 / INRA 1418, Ecologie Microbienne, Villeurbanne, France, 3 UMR 7266 CNRS-Université de la Rochelle, LIttoral ENvironnement Et Sociétés, La Rochelle, France, Edited by: 4 School of Mining and Geosciences, Nazarbayev University, Astana, Kazakhstan, 5 Museum National d’Histoire Naturelle, Christophe Dupraz, Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie, UMR 7590 CNRS Sorbonne Universités, Université Stockholm University, Sweden Pierre et Marie Curie, Institut de Recherche pour le Développement UMR 206, Paris, France, 6 UMR MARBEC, IRD, Ifremer, Reviewed by: CNRS, Université de Montpellier, Sète, France Trinity L. Hamilton, University of Minnesota Twin Cities, United States Lake Dziani Dzaha is a thalassohaline tropical crater lake located on the “Petite Terre” Virginia Helena Albarracín, Island of Mayotte (Comoros archipelago, Western Indian Ocean). Stromatolites are Center for Electron Microscopy (CIME), Argentina actively growing in the shallow waters of the lake shores. These stromatolites are *Correspondence: mainly composed of aragonite with lesser proportions of hydromagnesite, calcite, Emmanuelle Gérard dolomite, and phyllosilicates. -

Limibaculum Halophilum Gen. Nov., Sp. Nov., a New Member of the Family Rhodobacteraceae
TAXONOMIC DESCRIPTION Shin et al., Int J Syst Evol Microbiol 2017;67:3812–3818 DOI 10.1099/ijsem.0.002200 Limibaculum halophilum gen. nov., sp. nov., a new member of the family Rhodobacteraceae Yong Ho Shin,1 Jong-Hwa Kim,1 Ampaitip Suckhoom,2 Duangporn Kantachote2 and Wonyong Kim1,* Abstract A Gram-stain-negative, cream-pigmented, aerobic, non-motile, non-spore-forming and short-rod-shaped bacterial strain, designated CAU 1123T, was isolated from mud from reclaimed land. The strain’s taxonomic position was investigated by using a polyphasic approach. Strain CAU 1123T grew optimally at 37 C and at pH 7.5 in the presence of 2 % (w/v) NaCl. Phylogenetic analysis based on the 16S rRNA gene sequence revealed that strain CAU 1123T formed a monophyletic lineage within the family Rhodobacteraceae with 93.8 % or lower sequence similarity to representatives of the genera Rubrimonas, Oceanicella, Pleomorphobacterium, Rhodovulum and Albimonas. The major fatty acids were C18 : 1 !7c and 11-methyl C18 : 1 !7c and the predominant respiratory quinone was Q-10. The polar lipids were phosphatidylethanolamine, phosphatidylglycerol, two unidentified phospholipids, one unidentified aminolipid and one unidentified lipid. The DNA G+C content was 71.1 mol%. Based on the data from phenotypic, chemotaxonomic and phylogenetic studies, it is proposed that strain CAU 1123T represents a novel genus and novel species of the family Rhodobacteraceae, for which the name Limibaculumhalophilum gen. nov., sp. nov. The type strain is CAU 1123T (=KCTC 52187T, =NBRC 112522T). The family Rhodobacteraceae was first established by Garr- chemotaxonomic properties along with a detailed phyloge- ity et al. -

Detection of Microbial 16S Rrna Gene in the Blood of Patients with Parkinson’S Disease
ORIGINAL RESEARCH published: 24 May 2018 doi: 10.3389/fnagi.2018.00156 Detection of Microbial 16S rRNA Gene in the Blood of Patients With Parkinson’s Disease Yiwei Qian 1†, Xiaodong Yang 1†, Shaoqing Xu 1, Chunyan Wu 2, Nan Qin 2*, Sheng-Di Chen 1* and Qin Xiao 1* 1Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Department of Bioinformatics, Realbio Genomics Institute, Shanghai, China Emerging evidence suggests that the microbiota present in feces plays a role in Parkinson’s disease (PD). However, the alterations of the microbiome in the blood of PD patients remain unknown. To test this hypothesis, we conducted this case-control study to explore the microbiota compositions in the blood of Chinese PD patients. Microbiota communities in the blood of 45 patients and their healthy spouses were investigated using high-throughput Illumina HiSeq sequencing targeting the V3-V4 region of 16S ribosomal RNA (rRNA) gene. The relationships between the microbiota in the blood and Edited by: PD clinical characteristics were analyzed. No difference was detected in the structure Jiawei Zhou, and richness between PD patients and healthy controls. The following genera were Institute of Neuroscience, Shanghai Institutes for Biological Sciences enriched in the blood of PD patients: Isoptericola, Cloacibacterium, Enhydrobacter (CAS), China and Microbacterium; whereas genus Limnobacter was enriched in the healthy controls Reviewed by: after adjusting for age, gender, body mass index (BMI) and constipation. Additionally, Liu Zhihua, University of Chinese Academy of the findings regarding these genera were validated in another independent group of Sciences (UCAS), China 58 PD patients and 57 healthy controls using real-time PCR targeting genus-specific Shuo Yang, Nanjing Medical University, China 16S rRNA genes. -

Supplement of Biogeosciences, 16, 4229–4241, 2019 © Author(S) 2019
Supplement of Biogeosciences, 16, 4229–4241, 2019 https://doi.org/10.5194/bg-16-4229-2019-supplement © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement of Identifying the core bacterial microbiome of hydrocarbon degradation and a shift of dominant methanogenesis pathways in the oil and aqueous phases of petroleum reservoirs of different temperatures from China Zhichao Zhou et al. Correspondence to: Ji-Dong Gu ([email protected]) and Bo-Zhong Mu ([email protected]) The copyright of individual parts of the supplement might differ from the CC BY 4.0 License. 1 Supplementary Data 1.1 Characterization of geographic properties of sampling reservoirs Petroleum fluids samples were collected from eight sampling sites across China covering oilfields of different geological properties. The reservoir and crude oil properties together with the aqueous phase chemical concentration characteristics were listed in Table 1. P1 represents the sample collected from Zhan3-26 well located in Shengli Oilfield. Zhan3 block region in Shengli Oilfield is located in the coastal area from the Yellow River Estuary to the Bohai Sea. It is a medium-high temperature reservoir of fluvial face, made of a thin layer of crossed sand-mudstones, pebbled sandstones and fine sandstones. P2 represents the sample collected from Ba-51 well, which is located in Bayindulan reservoir layer of Erlian Basin, east Inner Mongolia Autonomous Region. It is a reservoir with highly heterogeneous layers, high crude oil viscosity and low formation fluid temperature. It was dedicated to water-flooding, however, due to low permeability and high viscosity of crude oil, displacement efficiency of water-flooding driving process was slowed down along the increase of water-cut rate. -

Ocean Acidification Reduces Induction of Coral Settlement by Crustose
Global Change Biology (2013) 19, 303–315, doi: 10.1111/gcb.12008 Ocean acidification reduces induction of coral settlement by crustose coralline algae NICOLE S. WEBSTER, SVEN UTHICKE, EMANUELLE S. BOTTE´ , FLORITA FLORES and ANDREW P. NEGRI Australian Institute of Marine Science, PMB 3, Townsville Mail Centre, Townsville, Qld 4810, Australia Abstract Crustose coralline algae (CCA) are a critical component of coral reefs as they accrete carbonate for reef structure and act as settlement substrata for many invertebrates including corals. CCA host a diversity of microorganisms that can also play a role in coral settlement and metamorphosis processes. Although the sensitivity of CCA to ocean acidificat- ion (OA) is well established, the response of their associated microbial communities to reduced pH and increased CO2 was previously not known. Here we investigate the sensitivity of CCA-associated microbial biofilms to OA and determine whether or not OA adversely affects the ability of CCA to induce coral larval metamorphosis. We experi- mentally exposed the CCA Hydrolithon onkodes to four pH/pCO2 conditions consistent with current IPCC predictions l for the next few centuries (pH: 8.1, 7.9, 7.7, 7.5, pCO2: 464, 822, 1187, 1638 atm). Settlement and metamorphosis of = l coral larvae was reduced on CCA pre-exposed to pH 7.7 (pCO2 1187 atm) and below over a 6-week period. Addi- tional experiments demonstrated that low pH treatments did not directly affect the ability of larvae to settle, but instead most likely altered the biochemistry of the CCA or its microbial associates. Detailed microbial community analysis of the CCA revealed diverse bacterial assemblages that altered significantly between pH 8.1 = l = l (pCO2 464 atm) and pH 7.9 (pCO2 822 atm) with this trend continuing at lower pH/higher pCO2 treatments. -
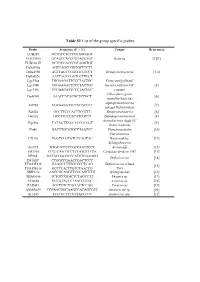
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Microbial Community Structure Analysis in Acer Palmatum Bark and Isolation of Novel Bacteria IAD-21 of the Phylum Abditibacteriota (Former Candidate Division FBP)
Microbial community structure analysis in Acer palmatum bark and isolation of novel bacteria IAD-21 of the phylum Abditibacteriota (former candidate division FBP) Kazuki Kobayashi1 and Hideki Aoyagi1,2 1 Division of Life Sciences and Bioengineering, Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan 2 Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan ABSTRACT Background: The potential of unidentified microorganisms for academic and other applications is limitless. Plants have diverse microbial communities associated with their biomes. However, few studies have focused on the microbial community structure relevant to tree bark. Methods: In this report, the microbial community structure of bark from the broad-leaved tree Acer palmatum was analyzed. Both a culture-independent approach using polymerase chain reaction (PCR) amplification and next generation sequencing, and bacterial isolation and sequence-based identification methods were used to explore the bark sample as a source of previously uncultured microorganisms. Molecular phylogenetic analyses based on PCR-amplified 16S rDNA sequences were performed. Results: At the phylum level, Proteobacteria and Bacteroidetes were relatively abundant in the A. palmatum bark. In addition, microorganisms from the phyla Acidobacteria, Gemmatimonadetes, Verrucomicrobia, Armatimonadetes, and Submitted 2 February 2019 Abditibacteriota, which contain many uncultured microbial species, existed in the Accepted 12 September 2019 A. palmatum bark. Of the 30 genera present at relatively high abundance in the bark, Published 29 October 2019 some genera belonging to the phyla mentioned were detected. A total of 70 isolates Corresponding author could be isolated and cultured using the low-nutrient agar media DR2A and Hideki Aoyagi, PE03. -

Supplementary Information
1 Supplementary information A B Control Vitiligo-NL Vitiligo-L Control Vitiligo-NL Vitiligo-L phylum genus Alphaproteobacteria Paracoccus Gammaproteobacteria Acinetobacter Deltaproteobacteria Haematobacter Brevundimonas Pararhizobium Enhydrobacter Uncultured E. Shigella Roseomonas Amaricoccus Methylobacterium Rhodobacteraceae Sphingomonas Aureimonas Undibacterium Luteimonas Haemophilus Moraxella Ochrobactrum Pseudomonas Acetobacter 2 3 4 SUPPLEMENTARY FIGURE 1 Enrichment of Gammaproteobacteria and Paracoccus in 5 skin swab samples from vitiligo patients 6 Composition and relative abundance of Proteobacteria between different groups was examined 7 at phylum (A) and genus (B) levels. Individual subjects are shown as taxa bar plots (above) and 8 grouped data as pie charts (below). A complete list of OTUs is shown in Supplementary Table 9 5. 10 1 11 12 13 SUPPLEMENTARY FIGURE 2 Differences in b-diversity between swab and biopsy samples 14 PCoA plot representing b-diversity between biopsy and swab samples from the lesional and 15 non-lesional sites of vitiligo patients compared to healthy controls. Microbiota profile in skin 16 biopsies is very different from skin swabs (P<0.001) and skin biopsies taken from lesional sites 17 of vitiligo patients (light green) are significantly different from all other samples (P<0.001). 18 19 20 21 22 23 24 25 2 Control Vitiligo NL Vitiligo L Staphylococcus Cutibacterium Mycoplasma Streptococcus Mitochondrial DNA Corynebacterium Intestinibacteria Bacteroides Clostridium Enterococcus Escherichia-Shigella Parabacteroides Veillonella Bifidobacterium Gemella Rothia Raistonia Undibacterium Uncultured bacteria Lactobacillus 26 27 28 SUPPLEMENTARY FIGURE 3 Composition and diversity of skin microbiota in healthy and 29 vitiligo skin biopsy samples 30 Individual subject data are shown at the genus level in form of a heat map, illustrating the top 31 20 of the most abundant bacterial taxa between the three groups (n=10 per group). -

Appendix 1. New and Emended Taxa Described Since Publication of Volume One, Second Edition of the Systematics
188 THE REVISED ROAD MAP TO THE MANUAL Appendix 1. New and emended taxa described since publication of Volume One, Second Edition of the Systematics Acrocarpospora corrugata (Williams and Sharples 1976) Tamura et Basonyms and synonyms1 al. 2000a, 1170VP Bacillus thermodenitrificans (ex Klaushofer and Hollaus 1970) Man- Actinocorallia aurantiaca (Lavrova and Preobrazhenskaya 1975) achini et al. 2000, 1336VP Zhang et al. 2001, 381VP Blastomonas ursincola (Yurkov et al. 1997) Hiraishi et al. 2000a, VP 1117VP Actinocorallia glomerata (Itoh et al. 1996) Zhang et al. 2001, 381 Actinocorallia libanotica (Meyer 1981) Zhang et al. 2001, 381VP Cellulophaga uliginosa (ZoBell and Upham 1944) Bowman 2000, VP 1867VP Actinocorallia longicatena (Itoh et al. 1996) Zhang et al. 2001, 381 Dehalospirillum Scholz-Muramatsu et al. 2002, 1915VP (Effective Actinomadura viridilutea (Agre and Guzeva 1975) Zhang et al. VP publication: Scholz-Muramatsu et al., 1995) 2001, 381 Dehalospirillum multivorans Scholz-Muramatsu et al. 2002, 1915VP Agreia pratensis (Behrendt et al. 2002) Schumann et al. 2003, VP (Effective publication: Scholz-Muramatsu et al., 1995) 2043 Desulfotomaculum auripigmentum Newman et al. 2000, 1415VP (Ef- Alcanivorax jadensis (Bruns and Berthe-Corti 1999) Ferna´ndez- VP fective publication: Newman et al., 1997) Martı´nez et al. 2003, 337 Enterococcus porcinusVP Teixeira et al. 2001 pro synon. Enterococcus Alistipes putredinis (Weinberg et al. 1937) Rautio et al. 2003b, VP villorum Vancanneyt et al. 2001b, 1742VP De Graef et al., 2003 1701 (Effective publication: Rautio et al., 2003a) Hongia koreensis Lee et al. 2000d, 197VP Anaerococcus hydrogenalis (Ezaki et al. 1990) Ezaki et al. 2001, VP Mycobacterium bovis subsp. caprae (Aranaz et al. -

A Time Travel Story: Metagenomic Analyses Decipher the Unknown Geographical Shift and the Storage History of Possibly Smuggled Antique Marble Statues
Annals of Microbiology (2019) 69:1001–1021 https://doi.org/10.1007/s13213-019-1446-3 ORIGINAL ARTICLE A time travel story: metagenomic analyses decipher the unknown geographical shift and the storage history of possibly smuggled antique marble statues Guadalupe Piñar1 & Caroline Poyntner1 & Hakim Tafer 1 & Katja Sterflinger1 Received: 3 December 2018 /Accepted: 30 January 2019 /Published online: 23 February 2019 # The Author(s) 2019 Abstract In this study, three possibly smuggled marble statues of an unknown origin, two human torsi (a female and a male) and a small head, were subjected to molecular analyses. The aim was to reconstruct the history of the storage of each single statue, to infer the possible relationship among them, and to elucidate their geographical shift. A genetic strategy, comprising metagenomic analyses of the 16S ribosomal DNA (rDNA) of prokaryotes, 18S rDNA of eukaryotes, as well as internal transcribed spacer regions of fungi, was performed by using the Ion Torrent sequencing platform. Results suggest a possible common history of storage of the two human torsi; their eukaryotic microbiomes showed similarities comprising many soil-inhabiting organisms, which may indicate storage or burial in land of agricultural soil. For the male torso, it was possible to infer the geographical origin, due to the presence of DNA traces of Taiwania, a tree found only in Asia. The small head displayed differences concerning the eukaryotic community, compared with the other two samples, but showed intriguing similarities with the female torso concerning the bacterial community. Both displayed many halotolerant and halophilic bacteria, which may indicate a longer stay in arid and semi-arid surroundings as well as marine environments.