The Decline and Conservation Status of North American Bumble Bees
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
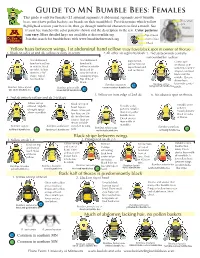
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

The Conservation Management and Ecology of Northeastern North
THE CONSERVATION MANAGEMENT AND ECOLOGY OF NORTHEASTERN NORTH AMERICAN BUMBLE BEES AMANDA LICZNER A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN BIOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2020 © Amanda Liczner, 2020 ii Abstract Bumble bees (Bombus spp.; Apidae) are among the pollinators most in decline globally with a main cause being habitat loss. Habitat requirements for bumble bees are poorly understood presenting a research gap. The purpose of my dissertation is to characterize the habitat of bumble bees at different spatial scales using: a systematic literature review of bumble bee nesting and overwintering habitat globally (Chapter 1); surveys of local and landcover variables for two at-risk bumble bee species (Bombus terricola, and B. pensylvanicus) in southern Ontario (Chapter 2); identification of conservation priority areas for bumble bee species in Canada (Chapter 3); and an analysis of the methodology for locating bumble bee nests using detection dogs (Chapter 4). The main findings were current literature on bumble bee nesting and overwintering habitat is limited and biased towards the United Kingdom and agricultural habitats (Ch.1). Bumble bees overwinter underground, often on shaded banks or near trees. Nests were mostly underground and found in many landscapes (Ch.1). B. terricola and B. pensylvanicus have distinct habitat characteristics (Ch.2). Landscape predictors explained more variation in the species data than local or floral resources (Ch.2). Among local variables, floral resources were consistently important throughout the season (Ch.2). Most bumble bee conservation priority areas are in western Canada, southern Ontario, southern Quebec and across the Maritimes and are most often located within woody savannas (Ch.3). -

The Rusty Patched Bumble Bee (Bombus Affinis) Voluntary Implementation Guidance for Section 10(A)(1)(B) of the Endangered Species Act
The Rusty Patched Bumble Bee (Bombus affinis) Voluntary Implementation Guidance for Section 10(a)(1)(B) of the Endangered Species Act Version 1.1 U.S. Fish & Wildlife Service, Regions 3, 4, 5 and 6 March 21, 2017 Contents Background and Purpose ................................................................................................................ 1 Current Versions of this Guidance .................................................................................................. 1 Range of Rusty Patched Bumble Bee .............................................................................................. 1 Brief Description of the Habitat Model ...................................................................................... 2 Section 10(a)(1)(B) of the Endangered Species Act and the Rusty Patched Bumble Bee .............. 5 Screening and Evaluation of Projects – A Stepwise Approach ................................................... 5 Step 1. Determine whether the rusty patched bumble bee is likely to be present in the project area. ............................................................................................................................ 5 Step 2 - Review the Project for its Potential to Incidentally Take the Species ....................... 8 Step 3 - Review Measures to Avoid Incidental Take of the Rusty Patched Bumble Bee ...... 14 Conservation Measures ............................................................................................................ 15 Restore and Maintain High Quality Habitat -

In Northeastern Oregon
Northwest Science Notes The purpose of Notes is to publish papers typically less than five pages long. No specific format or content is required for articles published as Notes, but all will be peer-reviewed and must be scientifically credible. Authors may contact the Editor about the suitability of manuscripts for this section. Sujaya Rao1, William P. Stephen, Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon 97331 Chiho Kimoto, Department of Fisheries and Wildlife, Oregon State University, Corvallis, Oregon 97331 and Sandra J. DeBano, Department of Fisheries and Wildlife, Hermiston Agricultural Research and Extension Center, Oregon State University, Hermiston, Oregon 97838. The Status of the ‘Red-Listed’ Bombus occidentalis (Hymenoptera: Apiformes) in Northeastern Oregon Abstract The western bumble bee, Bombus occidentalis, is included on the red list of bees by The Xerces Society. It was once a common bumble bee west of the Cascades but in the late 1990s it experienced a dramatic decline along coastal regions. The cause was speculated to be due to the introduction of pathogens from captive-bred bumble bees used for pollination of greenhouse crops. In extensive surveys conducted in western and southern Oregon, 10 individuals have been recorded since 2000. In this note, we report the collection of 49 individual B. occidentalis over two years in the Zumwalt Prairie Preserve of northeastern Oregon. This finding shows that B. occidentalis persists in northeastern regions of the Pacific Northwest, either because of geographic isolation from or potential resistance to the pathogens that decimated populations in the western part of the region. Further research is needed to determine its occurrence in other regions of its historical range to assess the extent of its decline. -

The Western Bumble Bee Was Once Commonly Found N R in the Western United States and Canada
Thanks to Dr. Robbin Thorp, UC Davis. UC Thorp, Robbin Dr. to Thanks Guide developed and illustrated by Elaine Evans, The Xerces Society. Xerces The Evans, Elaine by illustrated and developed Guide Bombus appositus Bombus Bombus morrisoni Bombus Funding for bumble bee conservation provided by the CS Fund. CS the by provided conservation bee bumble for Funding Bombus melanopygus Bombus Bombus bifarius Bombus Visit www.xerces.org/bumblebees for more information. more for www.xerces.org/bumblebees Visit Bombus occidentalis Bombus , please contact [email protected] contact please , find you If FOR INVERTEBRATE CONSERVATION INVERTEBRATE FOR S X T OCIETY ERCES HE B. occidentalis B. populations. remaining will use this information to promote conservation of of conservation promote to information this use will www.xerces.org/bumblebees Society and scientists studying bumble bee decline decline bee bumble studying scientists and Society will help document their current range. The Xerces Xerces The range. current their document help will P h o in recent years. Your efforts to search for this bee bee this for search to efforts Your years. recent in t o b y D e Found in the mountains and northern areas northern and mountains the in Found r Columbia to central California have nearly disappeared disappeared nearly have California central to Columbia r Bombus huntii Bombus mixtus Bombus i c k D historic range, but populations from southern British British southern from populations but range, historic i t c h still be found in northern and eastern parts of their their of parts eastern and northern in found be still b u United States and Canada. -

The Conservation Status of Bumble Bees of Canada, the USA, and Mexico
The Conservation Status of Bumble Bees of Canada, the USA, and Mexico Scott Hoffman Black, Rich Hatfield, Sarina Jepsen, Sheila Colla and Rémy Vandame Photo: Clay Bolt Importance of Bumble Bees There are 57 bumble bee species in Canada, US and Mexico. Hunt’s bumble bee; Bombus huntii , Photo: Clay Bolt Importance of Bumble Bees Important pollinator of many crops. Bombus sandersoni , Sanderson’s bumble bee Photo: Clay Bolt Importance of Bumble Bees Keystone pollinator in ecosystems. Yelllowheaded bumble bee, Bombus flavifrons Photo: Clay Bolt Bumble Bee Extinction Risk Assessment Network of 75+ bumble bee experts & specialists worldwide Goal: global bumble bee extinction risk assessment using consistent criteria Bumble Bee Extinction Risk Assessment IUCN Red List Criteria for Evaluating Extinction Risk • Used a database of 250,000+ specimen records (created from multiple data providers and compiled by Leif Richardson for Bumble Bees of North America) • Evaluate changes between recent (2002-2012) and historic (pre-2002): • Range (Extent of Occurrence) • Relative abundance • Persistence (50 km x 50 km grid cell occupancy) Sources: Hatfield et al. 2015 Bumble Bee Extinction Risk Assessment IUCN Red List Criteria for Evaluating Extinction Risk • Analyses informed application of Red List Categories; BBSG members provided review • This method was then adapted applied to South American and Mesoamerican bumble bees. Sources: Hatfield et al. 2015 Bumble Bee Extinction Risk Assessment Trilateral Region 6 4 5 28% of bumble bees Critically Endangered in Canada, the Endangered United States, and 7 Vulnerable Mexico are in an IUCN Threatened Near Threatened Category Least Concern 3 Data Deficient 32 Sources: Hatfield et al. -

Meet the Rare, Threatened and Endangered Insect Pollinators Of
NDSU EXTENSION E1977 Meet the Rare, Threatened and Endangered Jeremy Hemberger Johanna James-Heinze Insect Pollinators of North Dakota David Lowenstein, Consumer Horticulture Extension Educator, Michigan State University Nathaniel Walton, Consumer Horticulture Extension Educator, Michigan State University Patrick Beauzay, Integrated Pest Management Coordinator and Research Specialist, NDSU Veronica Calles-Torrez, Post-doctoral Scientist, NDSU Gerald Fauske, Insect Collection Manager and Research Specialist, NDSU David L. Cuthrell, Esther McGinnis, Extension Horticulturist, NDSU Michigan State University Janet Knodel, Extension Entomologist, NDSU Erik Runquist, Minnesota Zoo Why are some pollinators in decline? Rusty Patched Bumble Bee (Bombus affinis) and Nectar, pollen and habitat are three major requirements of Yellow-banded Bumble Bee (B. terricola) pollinators. When habitats (for example, natural areas) are lost to Gardeners frequently see and recognize bumble bees throughout agriculture, residential homes or commercial spaces, some insect the growing season, but some species have declined rapidly in the pollinators can undergo a rapid decline. Specialized pollinators are past few decades. Two declining bumble bee species are native to more susceptible to habitat or food losses because they often are the northern U.S. from the Dakotas eastward. dependent on a few specific host plants in a specialized habitat. Both feed on specific plants, compared with other bumble bees, Environmental contamination from using herbicides that which feed on a wider host plant list. In addition to habitat loss, prevent flowers from blooming or insecticides that kill pollinators their decline may be caused by a natural disadvantage in tolerating immediately or through time degrades otherwise suitable habitats. pathogens spread from commercially reared bumble bees. -

Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan Prepared By: Logan M. Rowe, David L. Cuthrell, and Helen D. Enander Michigan Natural Features Inventory Michigan State University Extension P.O. Box 13036 Lansing, MI 48901 Prepared For: Michigan Department of Natural Resources Wildlife Division 12/17/2019 MNFI Report No. 2019-33 Suggested Citation: Rowe, L. M., D. L. Cuthrell., H. D. Enander. 2019. Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan. Michigan Natural Features Inventory, Report Number 2019- 33, Lansing, USA. Copyright 2019 Michigan State University Board of Trustees. MSU Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover: Bombus terricola taken by D. L. Cuthrell Table of Contents Abstract ........................................................................................................................................................ iii Introduction .................................................................................................................................................. 1 Methods ........................................................................................................................................................ 2 Museum Searches .................................................................................................................................... -

Plos ONE 10(5): E0125847
RESEARCH ARTICLE Hitting an Unintended Target: Phylogeography of Bombus brasiliensis Lepeletier, 1836 and the First New Brazilian Bumblebee Species in a Century (Hymenoptera: Apidae) José Eustáquio Santos Júnior1, Fabrício R. Santos1, Fernando A. Silveira2* 1 Departamento de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2 Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil * [email protected] OPEN ACCESS Abstract Citation: Santos Júnior JE, Santos FR, Silveira FA (2015) Hitting an Unintended Target: Phylogeography This work tested whether or not populations of Bombus brasiliensis isolated on mountain of Bombus brasiliensis Lepeletier, 1836 and the First tops of southeastern Brazil belonged to the same species as populations widespread in low- New Brazilian Bumblebee Species in a Century (Hymenoptera: Apidae). PLoS ONE 10(5): e0125847. land areas in the Atlantic coast and westward along the Paraná-river valley. Phylogeo- doi:10.1371/journal.pone.0125847 graphic and population genetic analyses showed that those populations were all Academic Editor: Sean Brady, Smithsonian National conspecific. However, they revealed a previously unrecognized, apparently rare, and poten- Museum of Natural History, UNITED STATES tially endangered species in one of the most threatened biodiversity hotspots of the World, Received: September 18, 2014 the Brazilian Atlantic Forest. This species is described here as Bombus bahiensis sp. n., and included in a revised key for the identification of the bumblebee species known to occur Accepted: March 25, 2015 in Brazil. Phylogenetic analyses based on two mtDNA markers suggest this new species to Published: May 20, 2015 be sister to B. -

DNA Amplification from Pin-Mounted Bumble Bees (Bombus) in a Museum Collection: Effects of Fragment Size and Specimen Age on Successful PCR James P
DNA amplification from pin-mounted bumble bees (Bombus) in a museum collection: effects of fragment size and specimen age on successful PCR James P. Strange, Joyce Knoblett, Terry Griswold To cite this version: James P. Strange, Joyce Knoblett, Terry Griswold. DNA amplification from pin-mounted bumble bees (Bombus) in a museum collection: effects of fragment size and specimen age on successful PCR. Apidologie, Springer Verlag, 2009, 40 (2), pp.134-139. hal-00891957 HAL Id: hal-00891957 https://hal.archives-ouvertes.fr/hal-00891957 Submitted on 1 Jan 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie 40 (2009) 134–139 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2009 www.apidologie.org DOI: 10.1051/apido/2008070 Original article DNA amplification from pin-mounted bumble bees (Bombus) in a museum collection: effects of fragment size and specimen age on successful PCR* James P. Strange,JoyceKnoblett, Terry Griswold USDA-ARS, Bee Biology and Systematics Laboratory, Utah State University, BNR 255 Logan UT 84322-5310, USA Received 28 December 2007 – Revised 28 August 2008 – Accepted 15 November 2008 Abstract – Historic data in the form of pinned specimens in entomological collections offer the potential to determine trends in genetic diversity of bumble bees (Bombus). -

Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019
Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019 The PIs are most appreciative for identification assistance provided by: Arian Farid and Alan R. Franck, Director and former Director, resp., University of South Florida Herbarium, Tampa, FL; Edwin Bridges, Botanical and Ecological Consultant; Floyd Griffith, Botanist; and Eugene Wofford, Director, University of Tennessee Herbarium, Knoxville, TN Investigators Rick Johnstone and Robin Haggie (IVM Partners, 501-C-3 non-profit; http://www.ivmpartners.org/); Larry Porter and John Nettles (ret.), District 2 Wildflower Coordinator; Jeff Norcini, FDOT State Wildflower Specialist Cooperator Rick Owen (Imperiled Butterflies of Florida Work Group – North) Objective Evaluate a cost-effective strategy for creating habitat for pollinators/beneficial insects in the ROW beyond the back-slope. Rationale • Will aid FDOT in developing a strategy to create pollinator habitat per the federal BEE Act and FDOT’s Wildflower Program • Will demonstrate that FDOT can simultaneously • Create sustainable pollinator habitat in an economical and ecological manner • Reduce mowing costs • Part of national effort coordinated by IVM Partners, who has • Established or will establish similar projects on roadside or utility ROWS in Alabama, Arkansas, Maryland, New Mexico, Oklahoma, Idaho, Montana, Virginia, West Virginia, and Tennessee; studies previously conducted in Arizona, Delaware, Michigan, and New Jersey • Developed partnerships with US Fish & Wildlife Service, Army Corps of Engineers, US Geological Survey, New Jersey Institute of Technology, Rutgers University, Chesapeake Bay Foundation, Chesapeake Wildlife Heritage, The Navajo Nation, The Wildlife Habitat Council, The Pollinator Partnership, Progressive Solutions, Bayer Crop Sciences, Universities of Maryland, Ohio, West Virginia, and the EPA. -

Bumble Bees Are Essential
Prepared by the Bombus Task Force of the North American Pollinator Protection Campaign (NAPPC) Photo Sheila Colla Rusty-patched bumble bee, Bombus affi nis Declining North American Bumble Bees Photo David Inouye Western bumble bee, Bombus occidentalis Bumble Bees Helping Photo James Strange are Pollinators Essential Thrive Franklin bumble bee, Bombus franklini Bumble Bee Facts Photo Leif Richardson Globally, there are about 250 described species Some bumble bee are known to rob fl owers of bumble bees. They are found primarily in the of their nectar. Nectar robbing occurs when temperate zones of North and South America, a bee extracts nectar from a fl ower without and Eurasia. coming into contact with its reproductive Yellow-banded bumble bee, Bumble bees are documented to pollinate parts (i.e. anthers and/or stigma), usually Bombus terricola many important food crops. They are also more by biting a hole at the base of the fl ower. Photo Ron Hemberger effective than honey bees at pollinating crops Bumble bees are effective buzz pollinators grown in greenhouses. of several economically important plants in When most insects are inactive due to cold the family Solanaceae such as tomato, bell temperatures bumble bees are able to fl y by pepper and eggplant. In buzz pollination warming their fl ight muscles by shivering, bees extract pollen from a fl ower by American bumble bee, enabling them to raise their body temperature vibrating against the fl ower’s anthers, Bombus pensylvanicus as necessary for fl ight. making an audible buzzing noise. Instead of starting their own colonies, some Currently, the Common Eastern bumble bee bumble bee species have evolved to take over (Bombus impatiens) is the only species being another species’ colony to rear their young.