Interspecific Geographic Distribution and Variation of the Pathogens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thresholds of Response in Nest Thermoregulation by Worker Bumble Bees, Bombus Bifarius Nearcticus (Hymenoptera: Apidae)
Ethology 107, 387Ð399 (2001) Ó 2001 Blackwell Wissenschafts-Verlag, Berlin ISSN 0179±1613 Animal Behavior Area, Department of Psychology, University of Washington, Seattle and Department of Psychology, University of Puget Sound, Tacoma Thresholds of Response in Nest Thermoregulation by Worker Bumble Bees, Bombus bifarius nearcticus (Hymenoptera: Apidae) Sean O'Donnell & Robin L. Foster O'Donnell, S. & Foster, R. L. 2001: Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae). Ethology 107, 387Ð399. Abstract Regulation of nest temperature is important to the ®tness of eusocial insect colonies. To maintain appropriate conditions for the developing brood, workers must exhibit thermoregulatory responses to ambient temperature. Because nest- mate workers dier in task performance, thermoregulatory behavior provides an opportunity to test threshold of response models for the regulation of division of labor. We found that worker bumble bees (Bombus bifarius nearcticus) responded to changes in ambient temperature by altering their rates of performing two tasks ± wing fanning and brood cell incubation. At the colony level, the rate of incubating decreased, and the rate of fanning increased, with increasing temperature. Changes in the number of workers performing these tasks were more important to the colony response than changes in workers' task perform- ance rates. At the individual level, workers' lifetime rates of incubation and fanning were positively correlated, and most individuals did not specialize exclusively on either of these temperature-sensitive tasks. However, workers diered in the maximum temperature at which they incubated and in the minimum temperature at which they fanned. More individuals fanned at high and incubated at low temperatures. -

Interspecific Geographic Distribution and Variation of the Pathogens
Journal of Invertebrate Pathology xxx (2011) xxx–xxx Contents lists available at SciVerse ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations Nils Cordes a, Wei-Fone Huang b, James P. Strange c, Sydney A. Cameron d, Terry L. Griswold c, ⇑ Jeffrey D. Lozier e, Leellen F. Solter b, a University of Bielefeld, Evolutionary Biology, Morgenbreede 45, 33615 Bielefeld, Germany b Illinois Natural History Survey, Prairie Research Institute, University of Illinois, 1816 S. Oak St., Champaign, IL 61820, United States c USDA-ARS Pollinating Insects Research Unit, Utah State University, Logan, UT 84322-5310, United States d Department of Entomology and Institute for Genomic Biology, University of Illinois, Urbana, IL 61801, United States e Department of Biological Sciences, University of Alabama, Tuscaloosa, AL 35487, United States article info abstract Article history: Several bumble bee (Bombus) species in North America have undergone range reductions and rapid Received 4 September 2011 declines in relative abundance. Pathogens have been suggested as causal factors, however, baseline data Accepted 10 November 2011 on pathogen distributions in a large number of bumble bee species have not been available to test this Available online xxxx hypothesis. In a nationwide survey of the US, nearly 10,000 specimens of 36 bumble bee species collected at 284 sites were evaluated for the presence and prevalence of two known Bombus pathogens, the micros- Keywords: poridium Nosema bombi and trypanosomes in the genus Crithidia. Prevalence of Crithidia was 610% for all Bombus species host species examined but was recorded from 21% of surveyed sites. -
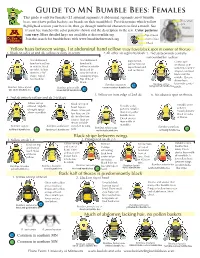
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Non-Leishmania Parasite in Fatal Visceral Leishmaniasis–Like Disease, Brazil
DISPATCHES Non-Leishmania Parasite in Fatal Visceral Leishmaniasis–Like Disease, Brazil Sandra R. Maruyama,1 Alynne K.M. de Santana,1,2 performed whole-genome sequencing of 2 clinical isolates Nayore T. Takamiya, Talita Y. Takahashi, from a patient with a fatal illness with clinical characteris- Luana A. Rogerio, Caio A.B. Oliveira, tics similar to those of VL. Cristiane M. Milanezi, Viviane A. Trombela, Angela K. Cruz, Amélia R. Jesus, The Study Aline S. Barreto, Angela M. da Silva, During 2011–2012, we characterized 2 parasite strains, LVH60 Roque P. Almeida,3 José M. Ribeiro,3 João S. Silva3 and LVH60a, isolated from an HIV-negative man when he was 64 years old and 65 years old (Table; Appendix, https:// Through whole-genome sequencing analysis, we identified wwwnc.cdc.gov/EID/article/25/11/18-1548-App1.pdf). non-Leishmania parasites isolated from a man with a fatal Treatment-refractory VL-like disease developed in the man; visceral leishmaniasis–like illness in Brazil. The parasites signs and symptoms consisted of weight loss, fever, anemia, infected mice and reproduced the patient’s clinical mani- festations. Molecular epidemiologic studies are needed to low leukocyte and platelet counts, and severe liver and spleen ascertain whether a new infectious disease is emerging that enlargements. VL was confirmed by light microscopic exami- can be confused with leishmaniasis. nation of amastigotes in bone marrow aspirates and promas- tigotes in culture upon parasite isolation and by positive rK39 serologic test results. Three courses of liposomal amphotericin eishmaniases are caused by ≈20 Leishmania species B resulted in no response. -

Response of Pollinators to the Tradeoff Between Resource Acquisition And
Oikos 000: 001–010, 2011 doi: 10.1111/j.1600-0706.2011.19910.x © 2011 The Authors. Oikos © 2011 Nordic Society Oikos Subject Editor: Koos Biesmierer. Accepted 25 July 2011 0 Response of pollinators to the tradeoff between resource 53 acquisition and predator avoidance 55 5 Ana L. Llandres, Eva De Mas and Miguel A. Rodríguez-Gironés 60 A. L. Llandres ([email protected]), E. De Mas and M. A. Rodríguez-Gironés, Dept of Functional and Evolutionary Ecology, Estación Experimental de Zonas Áridas (CSIC), Carretera de Sacramento, s/n, ES-04120, La Cañada de San Urbano, Almería, Spain. 10 65 Although the behaviour of animals facing the conflicting demands of increasing foraging success and decreasing predation risk has been studied in many taxa, the response of pollinators to variations in both factors has only been studied in isola- tion. We compared visit rates of two pollinator species, hoverflies and honeybees, to 40 Chrysanthemum segetum patches 15 in which we manipulated predation risk (patches with and without crab spiders) and nectar availability (rich and poor patches) using a full factorial design. Pollinators responded differently to the tradeoff between maximising intake rate and minimising predation risk: honeybees preferred rich safe patches and avoided poor risky patches while the number of hov- 70 erflies was highest at poor risky patches. Because honeybees were more susceptible to predation than hoverflies, our results suggest that, in the presence of competition for resources, less susceptible pollinators concentrate their foraging effort on 20 riskier resources, where competition is less severe. Crab spiders had a negative effect on the rate at which inflorescences were visited by honeybees. -

Bombus Vancouverensis Cresson, 1878 Common Name: Two Form Bumble Bee ELCODE: IIHYM24070 Taxonomic Serial No.: 714787
Alaska Natural Heritage Program Conservation Status Report Bombus vancouverensis Cresson, 1878 Common Name: Two Form Bumble Bee ELCODE: IIHYM24070 Taxonomic Serial No.: 714787 Synonyms: Pyrobombus bifarius Cresson, 1878; Bombus bifarius Cresson, 1878 Taxonomy Notes: Williams et al. (2014) report that DNA barcode data indicate this species has two lineages. However, the Barcode of Life Data System (BOLD) has all samples of this species (n = 163) grouped in a single BIN (BOLD:AAB4830). Ghisbain et al. (2020) concluded that B. bifarius sensu stricto does not occur in Alaska, and therefore all specimens are now B. vancouverensis and Sikes & Rykken (2020) concurred. Report last updated – November 2, 2020 Conservation Status G5 S3 Occurrences, Range Number of Occurrences: 20, number of museum records: 3,646 (Canadian National Collection, Illinois Natural History Survey, University of Alaska Museum Insect Collection, University of Alberta Museums, Koch et al. 2015). AK Range Extent: Bombus vancouverensis occurrences 359,987 km2; 4-km2 grid cells: 19. Found on the Seward Peninsula, and in Interior Alaska, the Matanuska Valley and the Copper River Basin. Also in Haines and Alaska-Canada Border at Stewart. North American Distribution: Alaska and south west Yukon Territory to British Columbia to the Pacific Northwest. In the Great Basin, Rocky Mountains, Arizona, New Mexico, Sierra Mountains and California Coast. Trends Trends are based on museum voucher collections of all Bombus species. Short-term trends are focus the past two decades (2000’s and 2010s), whereas long-term trends are based on all years. Data originate from museum voucher collections only and are summarized by decade. White bars indicate the number of voucher collections for the species. -

Meet the Rare, Threatened and Endangered Insect Pollinators Of
NDSU EXTENSION E1977 Meet the Rare, Threatened and Endangered Jeremy Hemberger Johanna James-Heinze Insect Pollinators of North Dakota David Lowenstein, Consumer Horticulture Extension Educator, Michigan State University Nathaniel Walton, Consumer Horticulture Extension Educator, Michigan State University Patrick Beauzay, Integrated Pest Management Coordinator and Research Specialist, NDSU Veronica Calles-Torrez, Post-doctoral Scientist, NDSU Gerald Fauske, Insect Collection Manager and Research Specialist, NDSU David L. Cuthrell, Esther McGinnis, Extension Horticulturist, NDSU Michigan State University Janet Knodel, Extension Entomologist, NDSU Erik Runquist, Minnesota Zoo Why are some pollinators in decline? Rusty Patched Bumble Bee (Bombus affinis) and Nectar, pollen and habitat are three major requirements of Yellow-banded Bumble Bee (B. terricola) pollinators. When habitats (for example, natural areas) are lost to Gardeners frequently see and recognize bumble bees throughout agriculture, residential homes or commercial spaces, some insect the growing season, but some species have declined rapidly in the pollinators can undergo a rapid decline. Specialized pollinators are past few decades. Two declining bumble bee species are native to more susceptible to habitat or food losses because they often are the northern U.S. from the Dakotas eastward. dependent on a few specific host plants in a specialized habitat. Both feed on specific plants, compared with other bumble bees, Environmental contamination from using herbicides that which feed on a wider host plant list. In addition to habitat loss, prevent flowers from blooming or insecticides that kill pollinators their decline may be caused by a natural disadvantage in tolerating immediately or through time degrades otherwise suitable habitats. pathogens spread from commercially reared bumble bees. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Sarah A. Maxfield-Taylor for the degree of Master of Science in Entomology presented on March 26, 2014. Title: Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon Abstract approved: _____________________________________________ Sujaya U. Rao Bumble bees (Hymenoptera: Apidae) are important native pollinators in wild and agricultural systems, and are one of the few groups of native bees commercially bred for use in the pollination of a range of crops. In recent years, declines in bumble bees have been reported globally. One factor implicated in these declines, believed to affect bumble bee colonies in the wild and during rearing, is natural enemies. A diversity of fungi, protozoa, nematodes, and parasitoids has been reported to affect bumble bees, to varying extents, in different parts of the world. In contrast to reports of decline elsewhere, bumble bees have been thriving in Oregon on the West Coast of the U.S.A.. In particular, the agriculturally rich Willamette Valley in the western part of the state appears to be fostering several species. Little is known, however, about the natural enemies of bumble bees in this region. The objectives of this thesis were to: (1) identify pathogens and parasites in (a) bumble bees from the wild, and (b) bumble bees reared in captivity and (2) examine the effects of disease on bee hosts. Bumble bee queens and workers were collected from diverse locations in the Willamette Valley, in spring and summer. Bombus mixtus, Bombus nevadensis, and Bombus vosnesenskii collected from the wild were dissected and examined for pathogens and parasites, and these organisms were identified using morphological and molecular characteristics. -

Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington
Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington Final report from the Xerces Society to the U.S. Forest Service and Interagency Special Status/Sensitive Species Program (ISSSSP) Agreement L13AC00102, Modification 5 Bombus vosnesenskii on Balsamorhiza sagittata. Photo by Rich Hatfield, the Xerces Society. By Rich Hatfield, Sarina Jepsen, and Scott Black, the Xerces Society for Invertebrate Conservation September 2017 1 Table of Contents Abstract ......................................................................................................................................................... 3 Introduction .................................................................................................................................................. 3 Methods ........................................................................................................................................................ 6 Site Selection ............................................................................................................................................. 6 Site Descriptions (west to east) ................................................................................................................ 7 T14ES27 (USFS) ..................................................................................................................................... 7 Cape Horn (USFS) ................................................................................................................................. -

Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan Prepared By: Logan M. Rowe, David L. Cuthrell, and Helen D. Enander Michigan Natural Features Inventory Michigan State University Extension P.O. Box 13036 Lansing, MI 48901 Prepared For: Michigan Department of Natural Resources Wildlife Division 12/17/2019 MNFI Report No. 2019-33 Suggested Citation: Rowe, L. M., D. L. Cuthrell., H. D. Enander. 2019. Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan. Michigan Natural Features Inventory, Report Number 2019- 33, Lansing, USA. Copyright 2019 Michigan State University Board of Trustees. MSU Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover: Bombus terricola taken by D. L. Cuthrell Table of Contents Abstract ........................................................................................................................................................ iii Introduction .................................................................................................................................................. 1 Methods ........................................................................................................................................................ 2 Museum Searches .................................................................................................................................... -

New Approaches to Systematics of Trypanosomatidae
Opinion New Approaches to Systematics of Trypanosomatidae: Criteria for Taxonomic (Re)description 1,2 3 4 Jan Votýpka, Claudia M. d'Avila-Levy, Philippe Grellier, 5 ̌2,6,7 Dmitri A. Maslov, Julius Lukes, and 8,2,9, Vyacheslav Yurchenko * While dixenous trypanosomatids represent one of the most dangerous patho- Trends gens for humans and domestic animals, their monoxenous relatives have fre- The protists classified into the family quently become model organisms for studies of diversity of parasitic protists Trypanosomatidae (Euglenozoa: Kine- toplastea) represent a diverse and and host–parasite associations. Yet, the classification of the family Trypano- important group of organisms. somatidae is not finalized and often confusing. Here we attempt to make a Despite recent advances, the taxon- blueprint for future studies in this field. We would like to elicit a discussion about omy and systematics of Trypanosoma- an updated procedure, as traditional taxonomy was not primarily designed to be tidae are far from being consistent with used for protists, nor can molecular phylogenetics solve all the problems alone. the known phylogenetic affinities within this group. The current status, specific cases, and examples of generalized solutions are presented under conditions where practicality is openly favored over rigid We are eliciting a discussion about an taxonomic codes or blind phylogenetic approach. updated procedure in trypanosomatid systematics, as traditional taxonomy was not primarily designed to be used Classification of Trypanosomatids for -

Western Bumble Bee,Bombus Occidentalis
COSEWIC Assessment and Status Report on the Western Bumble Bee Bombus occidentalis occidentalis subspecies - Bombus occidentalis occidentalis mckayi subspecies - Bombus occidentalis mckayi in Canada occidentalis subspecies - THREATENED mckayi subspecies - SPECIAL CONCERN 2014 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2014. COSEWIC assessment and status report on the Western Bumble Bee Bombus occidentalis, occidentalis subspecies (Bombus occidentalis occidentalis) and the mckayi subspecies (Bombus occidentalis mckayi) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 52 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Production note: COSEWIC would like to acknowledge Sheila Colla, Michael Otterstatter, Cory Sheffield and Leif Richardson for writing the status report on the Western Bumble Bee, Bombus occidentalis, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Jennifer Heron, Co-chair of the COSEWIC Arthropods Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bourdon de l'Ouest (Bombus occidentalis) de la sous-espèce occidentalis (Bombus occidentalis occidentalis) et la sous-espèce mckayi (Bombus occidentalis mckayi) au Canada. Cover illustration/photo: Western Bumble Bee — Cover photograph by David Inouye, Western Bumble Bee worker robbing an Ipomopsis flower. Her Majesty the Queen in Right of Canada, 2014.